We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox1996
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structure == | == Structure == | ||
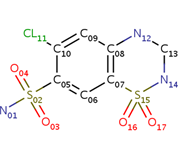
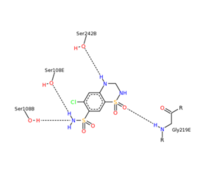
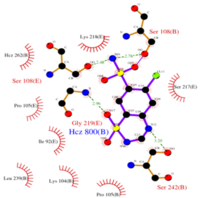
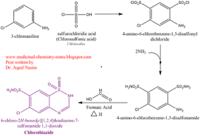
| - | Chlorothiazide is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) (reference). Its chemical formula is C7H8ClN3O4S2 and its molecular weight is of 298Da. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celcius, a solubility of 60 mg/m in DMSO and less than 1 mg/ml in water, and appears a white crystalline powder(reference). The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms (reference). <scene name='75/756546/Drug/1'>Cholorothiazide was determined when bound to glutamate receptor 2 </scene>through hydrogen bonding between the nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E (Figure 2 & 3). Synthesis of chlorothiazide occurs through the reaction between 3 -chloroaniline, chlorosulfonic acid, and ammonia; and | + | Chlorothiazide is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) (reference). Its chemical formula is C7H8ClN3O4S2 and its molecular weight is of 298Da. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celcius, a solubility of 60 mg/m in DMSO and less than 1 mg/ml in water, and appears a white crystalline powder(reference). The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms (reference). <scene name='75/756546/Drug/1'>Cholorothiazide was determined when bound to glutamate receptor 2 </scene>through hydrogen bonding between the nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E (Figure 2 & 3). Synthesis of chlorothiazide occurs through the reaction between 3 -chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 4)(reference). |
<center>[[Image:Duiril.png|thumb|left|200px|Figure 1. Molecule structure of chlorothiazide.]]</center> | <center>[[Image:Duiril.png|thumb|left|200px|Figure 1. Molecule structure of chlorothiazide.]]</center> | ||
| Line 41: | Line 41: | ||
===Kidney Stones=== | ===Kidney Stones=== | ||
| - | + | Kidneys are needed to filter fluids and waste from the body to produce urine. Sometimes there are high levels of chemicals in the urine that form crystals and the crystals eventually become large enough to form stones in the kidney <ref name = "three"> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </ref>. Chlorothiazide can help prevent against calcium kidney stones with patients that have high calcium concentrations in their blood.<ref name = "two"> AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html | |
</ref> Thiazides (diuril is an example of one) can cause potassium loss as well, which reduces citrate levels. Lowered citrate levels can increase the risk of kidney stones, so diuril needs to be taken in conjunction with potassium-citrate pills <ref name = "three"> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </ref>. | </ref> Thiazides (diuril is an example of one) can cause potassium loss as well, which reduces citrate levels. Lowered citrate levels can increase the risk of kidney stones, so diuril needs to be taken in conjunction with potassium-citrate pills <ref name = "three"> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </ref>. | ||
===Side Effects=== | ===Side Effects=== | ||
| - | One main side effect that should be noted when taking Diuril is the introduction of a | + | One main side effect that should be noted when taking Diuril is the introduction of a purpura, or excessive bruising and superficial bleeding typically on the legs <ref name = "eight"> The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224 </ref>. There are many different types of purpura, but the general bleeding of small vessels and inflammation hold true. Some patients, when taking Diuril more regularly, as in twice a day for a specific number of weeks, will exhibit this purpura. This can be treated typically with bedrest, minor medications, and discontinued use of chlorothiazide. One study from the Mayo Clinic showed that, after discontinuing use of chlorothiazide and then readministering a single dose, purpura reappeared quite rapidly, leading to inferences that chlorothiazide use was in fact what brought on the purpura originally <ref name = "nine"> Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265. </ref>. |
Revision as of 23:15, 29 March 2017
Diuril (Chlorothiazide)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794.
- ↑ 3.0 3.1 Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html
- ↑ RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103
- ↑ Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743.
- ↑ 6.0 6.1 Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones
- ↑ AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html
- ↑ The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224
- ↑ Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265.