IntronA (Interferon alpha 2b)
From Proteopedia
| Line 1: | Line 1: | ||
==An overview of IntronA (Interferon alpha 2b)== | ==An overview of IntronA (Interferon alpha 2b)== | ||
| + | |||
| + | IntronA, also known as interferon alpha-2b (IFNα2b), is a drug used in antiviral and anti-tumor therapeutic treatments <ref name="one"> doi: 10.1155/2014/970315</ref>. IFNα2b is classified as a cytokine, a secreted protein that stimulates the immune system, and is produced by T-cells in order to hinder viral infections, cancer, bacteria, or other pathogens in humans <ref name="one"> doi: 10.1155/2014/970315</ref>. | ||
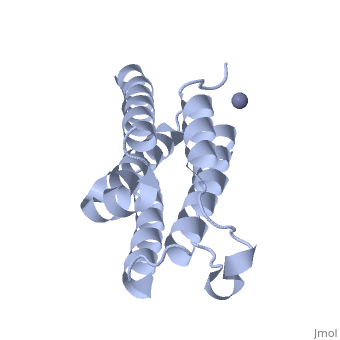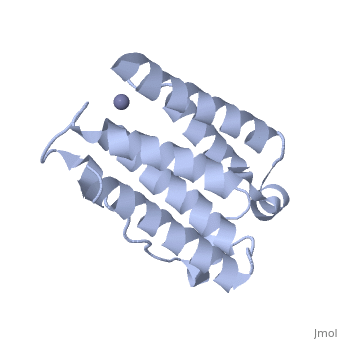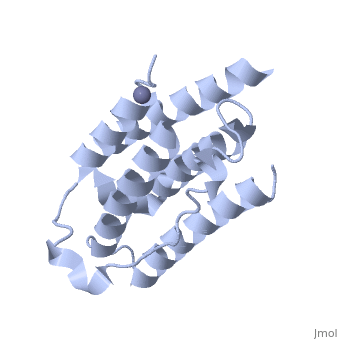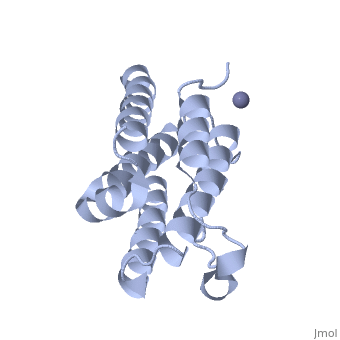
<StructureSection load='1rh2' size='340' side='right' caption='The IntronA (Interferon-alpha 2b) human recombinant' scene=''> | <StructureSection load='1rh2' size='340' side='right' caption='The IntronA (Interferon-alpha 2b) human recombinant' scene=''> | ||
| Line 7: | Line 9: | ||
== Function == | == Function == | ||
| - | == | + | IFNα2b is a naturally secreted protein that is produced by the human body via antigen-presenting cells (APCs) <ref name="two"> doi: 10.1089/107999099313325</ref>. When introduced to the body for treatment, IFNα2b is injected into the body subcutaneously and binds to the surface of cells <ref name="three"> https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf</ref> When this occurs, IFNα2b produces many possible outcomes. This includes inhibiting replication of viruses within virus-infected cells, suppressing cell proliferation, enhancing activity of macrophages, stimulating certain types of enzymes, and increasing lymphocytes’ specific cytotoxicity.3 Specifically, in viral infections and malignancy the interferon specifically targets CD8+ effector T cells and CD4+ immunomodulatory T cells to amplify the body’s immune response <ref name="two"> doi: 10.1089/107999099313325</ref>. It can also help induce a caspase cascade to activate cell death in virally infected and malignant cells <ref name="two"> doi: 10.1089/107999099313325</ref>. Overall, INFα2b is crucial for activation and regulation of the protective immune response. |
== Structural highlights == | == Structural highlights == | ||
| + | |||
| + | Each IFNα2b monomer consists of five alpha helices and five coiled loops.4 A zinc molecule is held within each IFNα2b monomer.4 There are four significant cysteine residues on IFNα2b that create two disulfide bonds <ref name="one"> doi: 10.1155/2014/970315</ref>. The first disulfide bond is between Cys29 and Cys138.1 The second disulfide bond is between Cys1 and Cys98 <ref name="one"> doi: 10.1155/2014/970315</ref>. IFNα2b is considered a Type I interferon and recognizes interferon alpha receptors 1 and 2 located on the target protein’s surface <ref name="one"> doi: 10.1155/2014/970315</ref>. Amino acid residues on IFNα2b that participate in binding to IFNAR 1 and 2 are present on one coiled loop and four alpha helices <ref name="one"> doi: 10.1155/2014/970315</ref>. These residues include: Arg22, Leu26, Phe27, Leu30, Lys31, Arg33, His34, Ser68, Thr79, Lys83, Tyr85, Tyr89, Arg120, Lys121, Gln124, Lys131 Glu132, Arg144, and Glu146 <ref name="one"> doi: 10.1155/2014/970315</ref>. | ||
== Mechanism == | == Mechanism == | ||
| + | There are several pathways in that IFNα2b has an effect on the target cell <ref name="one"> doi: 10.1155/2014/970315</ref>. These pathways include the caspase cascade and the JAK-STAT pathway <ref name="one"> doi: 10.1155/2014/970315</ref> <ref name="two"> doi: 10.1089/107999099313325</ref>. The caspase cascade results in apoptosis, thereby participating in both anti-viral and anti-cancer mechanisms <ref name="two"> doi: 10.1089/107999099313325</ref>. Once IFNα2b binds to either IFNAR 1 or 2 receptors, cytochrome c and tumor necrosis alpha factor trigger the caspase cascade, which in turn signals apoptosis <ref name="one"> doi: 10.1155/2014/970315</ref>. In the binding of IFNα2b to an IFNAR receptor, the protein JAK (a tyrosine kinase) is activated <ref name="one"> doi: 10.1155/2014/970315</ref>. JAK is phosphorylated and in turn phosphorylates the IFNAR receptors <ref name="one"> doi: 10.1155/2014/970315</ref>. The IFNAR receptors bind to STAT proteins resulting in a cascade pathway that signals the release of antiviral proteins <ref name="one"> doi: 10.1155/2014/970315</ref>. | ||
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| + | |||
| + | == Disease == | ||
| + | |||
| + | '''Hepatitis Virus''' | ||
| + | |||
| + | ''Hepatitis C'' | ||
| + | |||
| + | Hepatitis C (HCV) can range from acute to chronic conditions and progression occurs in more than half of these cases.5 HCV attacks the liver tissues causing inflammation and cirrhosis, which can lead to chronic liver disease.5 IFNα2b is used to treat the acute stage of HVC to prevent progression into the chronic disease.5 IFNα2b overall reduces the viral amplification and allows T-cells to effectively respond to the infection.6 | ||
| + | |||
| + | ''Hepatitis B'' | ||
| + | |||
| + | IFNα2b treats chronic Hepatitis B (HBV) by interfering with viral DNA synthesis and enhancing the cellular immune response.7 The cytokine enhances T-cell and natural killer cell activity by interacting with the infected cell’s surface.7 IFNα2b also activates antiviral enzymes to inhibit proliferation of HBV.7 | ||
| + | |||
| + | '''Cancer''' | ||
| + | |||
| + | ''Multiple Myeloma'' | ||
| + | |||
| + | IFNα2b has been shown to prolong patients with multiple myeloma.8 Multiple myeloma is diagnosed when multiple clones of a specific plasma cell are found or when apparent genetic mutations are affecting the cell’s normal functions.8 IFNα2b therapy causes tumor stabilization and prolongation of the aggressive stage of multiple myeloma with maintained therapy.8 | ||
| + | |||
| + | ''Hairy cell Leukemia'' | ||
| + | |||
| + | IFNα2b is a major treatment for patients with hairy cell leukemia <ref name="one"> doi: 10.1155/2014/970315</ref>. The disease is characterized by B cells in the blood, bone marrow, and spleen <ref name="one"> doi: 10.1155/2014/970315</ref>. Patients who receive the therapeutic treatment experience partial to complete remission <ref name="one"> doi: 10.1155/2014/970315</ref>. The treatment is especially effective because there is rarely relapse of the cancer with consistent therapy and mild side effects <ref name="one"> doi: 10.1155/2014/970315</ref>. The presence of malignant cells decreases, predominantly in the bone marrow, and hematologic levels normalized.9 | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | Ningrum A. R., & Ratih. (2014). Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica, 2014, 970315. doi: 10.1155/2014/970315 | ||
| + | |||
| + | Tompkins, W. A. (1999). Immunomodulation and Therapeutic Effects of the Oral Use of Interferon-alpha: Mechanism of Action. Journal of Interferon & Cytokine Research, 19(8), 817–828. doi: 10.1089/107999099313325 | ||
| + | |||
| + | Merck. (n.d.). Product information intron A interferon alfa-2b, recombinant for injection. Retrieved from https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf. | ||
| + | |||
| + | Radhakrishnan, R., Walter, L. J., Hruza, A., Reichert, P., Trotta, P. P., Nagabhushan, T. L., & Walter, M. R. (1996). Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure (London, England : 1993), 4(12), 1453–63. | ||
| + | |||
| + | Jaeckel, E., Cornberg, M., Wedemeyer, H., Santantonio, T., Mayer, J., Zankel, M., Pastore, G., Dietrich, M., Trautwein, C. & Manns, M. P. (2001). Treatment of Acute Hepatitis C with Interferon Alfa-2b. New England Journal of Medicine, 345(20), 1452–1457. doi: 10.1056/NEJMoa011232 | ||
| + | |||
| + | Guo, J.T., Sohn, A.J., Zhu, Q. & Seeger, C. (2004). Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology, 35 (1), 71-81. Doi: 10.1016/j.virol.2004.04.031 | ||
| + | |||
| + | Asselah, T., Lada, O., Moucari, R., Le Martinot, M., Boyer, N., & Marcellin, P. (n.d.). Interferon Therapy for Chronic Hepatitis B. doi: 10.1016/j.cld.2007.08.010 | ||
| + | |||
| + | Bladé, J., San Miguel, J., Escudero, M., Fontanillas, M., Besalduch, J., Gardella, S., Arias, J., Garcia-Conte, J., Carnero, M., Marti, J.M., Rozman, C., Estape, J. & Montserrat, E. (1998). Maintenance treatment with interferon alpha-2b in multiple myeloma:a prospective randomized study from PETHEMA ( Program for the Study and Treatment of Hematological Malignancies , Spanish Society of Hematology ). Leukemia, 12, 1144–1148. doi: 10.1038/sj.leu.2401039 | ||
| + | |||
| + | Quesada, J. R., Reuben, J., Manning, J. T., Hersh, E. M., & Gutterman, J. U. (1984). Alpha Interferon for Induction of Remission in Hairy-cell Leukemia. The New England Journal of Medicine Downloaded from Nejm.org at JAMES MADISON UNIV on, 310(1), 15–18. | ||
Revision as of 05:45, 30 March 2017
An overview of IntronA (Interferon alpha 2b)
IntronA, also known as interferon alpha-2b (IFNα2b), is a drug used in antiviral and anti-tumor therapeutic treatments [1]. IFNα2b is classified as a cytokine, a secreted protein that stimulates the immune system, and is produced by T-cells in order to hinder viral infections, cancer, bacteria, or other pathogens in humans [1].
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Asmana Ningrum R. Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica (Cairo). 2014;2014:970315. doi: 10.1155/2014/970315. Epub 2014 Mar, 10. PMID:24741445 doi:http://dx.doi.org/10.1155/2014/970315
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 4.0 4.1 4.2 4.3 4.4 Tompkins WA. Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res. 1999 Aug;19(8):817-28. PMID:10476925 doi:http://dx.doi.org/10.1089/107999099313325
- ↑ https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf
Ningrum A. R., & Ratih. (2014). Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica, 2014, 970315. doi: 10.1155/2014/970315
Tompkins, W. A. (1999). Immunomodulation and Therapeutic Effects of the Oral Use of Interferon-alpha: Mechanism of Action. Journal of Interferon & Cytokine Research, 19(8), 817–828. doi: 10.1089/107999099313325
Merck. (n.d.). Product information intron A interferon alfa-2b, recombinant for injection. Retrieved from https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf.
Radhakrishnan, R., Walter, L. J., Hruza, A., Reichert, P., Trotta, P. P., Nagabhushan, T. L., & Walter, M. R. (1996). Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure (London, England : 1993), 4(12), 1453–63.
Jaeckel, E., Cornberg, M., Wedemeyer, H., Santantonio, T., Mayer, J., Zankel, M., Pastore, G., Dietrich, M., Trautwein, C. & Manns, M. P. (2001). Treatment of Acute Hepatitis C with Interferon Alfa-2b. New England Journal of Medicine, 345(20), 1452–1457. doi: 10.1056/NEJMoa011232
Guo, J.T., Sohn, A.J., Zhu, Q. & Seeger, C. (2004). Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology, 35 (1), 71-81. Doi: 10.1016/j.virol.2004.04.031
Asselah, T., Lada, O., Moucari, R., Le Martinot, M., Boyer, N., & Marcellin, P. (n.d.). Interferon Therapy for Chronic Hepatitis B. doi: 10.1016/j.cld.2007.08.010
Bladé, J., San Miguel, J., Escudero, M., Fontanillas, M., Besalduch, J., Gardella, S., Arias, J., Garcia-Conte, J., Carnero, M., Marti, J.M., Rozman, C., Estape, J. & Montserrat, E. (1998). Maintenance treatment with interferon alpha-2b in multiple myeloma:a prospective randomized study from PETHEMA ( Program for the Study and Treatment of Hematological Malignancies , Spanish Society of Hematology ). Leukemia, 12, 1144–1148. doi: 10.1038/sj.leu.2401039
Quesada, J. R., Reuben, J., Manning, J. T., Hersh, E. M., & Gutterman, J. U. (1984). Alpha Interferon for Induction of Remission in Hairy-cell Leukemia. The New England Journal of Medicine Downloaded from Nejm.org at JAMES MADISON UNIV on, 310(1), 15–18.