User:Natalie Van Ochten/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
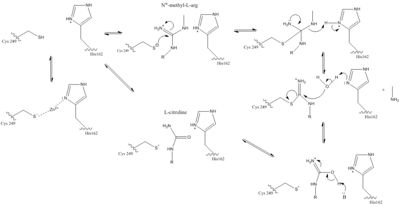
| - | Dimethylarginine Dimethyaminohydrolase (commonly known as DDAH) is a member of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Hydrolase hydrolase]</span> family of enzymes which use water to break down molecules <ref name="palm">Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/17933965 17933965]</span> doi:<span class="plainlinks">[http://ajpheart.physiology.org/content/293/6/H3227 10.1152/ajpheart.00998.2007]</span></ref>. Specifically, DDAH is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase nitric oxide synthase (NOS)]</span> regulator. Its metabolizes free arginine derivatives, namely <span class="plainlinks">[https://en.wikipedia.org/wiki/Asymmetric_dimethylarginine NѠ,NѠ-dimethyl-L-arginine (ADMA)]</span> and <span class="plainlinks">[https://en.wikipedia.org/wiki/Methylarginine NѠ-methyl-L-arginine (MMA)]</span> which competitively inhibit NOS <ref name="tran">Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14664901 14664901]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S1567568803000321 10.1016/S1567-5688(03)00032-1]</span></ref>. DDAH converts MMA and ADMA to <span class="plainlinks">[https://en.wikipedia.org/wiki/Citrulline L-citrulline]</span> and monoamine or dimethylamine <ref name="frey">Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16698551 16698551]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0969212606001717 10.1016/j.str.2006.03.006]</span></ref>. DDAH is expressed in the cytosol of cells in humans, mice, rates, sheep, cattle, and bacteria <ref name="palm" />. DDAH activity has been localized mainly to the brain, kidney, pancreas, and liver in these organisms. If DDAH is overexpressed, NOS can be activated <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of NOS (endothelial, neuronal, and inducible) <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. In humans, many diseases can come from improper control of NO levels including <span class="plainlinks">[https://en.wikipedia.org/wiki/Diabetes_mellitus diabetes mellitus]</span> and <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/high-blood-pressure/basics/definition/con-20019580 hypertension]</span>. Current research has identified several inhibitors of DDAH which could be important in fighting diseases involving irregular NO levels <ref name="frey" />. | + | Dimethylarginine Dimethyaminohydrolase (commonly known as DDAH) is a member of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Hydrolase hydrolase]</span> family of enzymes which use water to break down molecules <ref name="palm">Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/17933965 17933965]</span> doi:<span class="plainlinks">[http://ajpheart.physiology.org/content/293/6/H3227 10.1152/ajpheart.00998.2007]</span></ref>. Specifically, DDAH is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase nitric oxide synthase (NOS)]</span> regulator. Its metabolizes free arginine derivatives, namely <span class="plainlinks">[https://en.wikipedia.org/wiki/Asymmetric_dimethylarginine NѠ,NѠ-dimethyl-L-arginine (ADMA)]</span> and <span class="plainlinks">[https://en.wikipedia.org/wiki/Methylarginine NѠ-methyl-L-arginine (MMA)]</span> which competitively inhibit NOS <ref name="tran">Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14664901 14664901]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S1567568803000321 10.1016/S1567-5688(03)00032-1]</span></ref>. DDAH converts MMA and ADMA to <span class="plainlinks">[https://en.wikipedia.org/wiki/Citrulline L-citrulline]</span> and monoamine or dimethylamine <ref name="frey">Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16698551 16698551]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0969212606001717 10.1016/j.str.2006.03.006]</span></ref>. DDAH is expressed in the cytosol of cells in humans, mice, rates, sheep, cattle, and bacteria <ref name="palm" />. DDAH activity has been localized mainly to the brain, kidney, pancreas, and liver in these organisms. If DDAH is overexpressed, NOS can be activated <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase NOS (endothelial, neuronal, and inducible)]</span> <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. In humans, many diseases can come from improper control of NO levels including <span class="plainlinks">[https://en.wikipedia.org/wiki/Diabetes_mellitus diabetes mellitus]</span> and <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/high-blood-pressure/basics/definition/con-20019580 hypertension]</span>. Current research has identified several inhibitors of DDAH which could be important in fighting diseases involving irregular NO levels <ref name="frey" />. |
==General Structure== | ==General Structure== | ||
| Line 33: | Line 33: | ||
===Different Isoforms=== | ===Different Isoforms=== | ||
| - | DDAH has two main isoforms <ref name="frey" />. DDAH-1 colocalizes with | + | DDAH has two main isoforms <ref name="frey" />. DDAH-1 colocalizes with nNOS (neuronal NOS). This enzyme is found mainly in the brain and kidney of organisms <ref name="tran" />. DDAH-2 is found in tissues with eNOS (endothelial NOS) <ref name="frey" />. DDAH-2 localization has been found in the heart, kidney, and placenta <ref name="tran" />. Additionally, studies show that DDAH-2 is expressed in iNOS containing immune tissues (inducible NOS) <ref name="frey" />. Both of the isoforms have conserved residues that are involved in the catalytic mechanism of DDAH (Cys, Asp, and His). The differences between the isoforms is in the substrate binding residues and the lid region residues. DDAH-1 has a positively charged lid region while DDAH-2 has negatively charged lid. In total, three salt bridge differ between DDAH-1 and DDAH-2 isoforms. Researchers can take advantage of the fact that there are two different isoforms of this enzyme and create drugs that target one isoform over another to control NO levels in specific tissues in the body <ref name="frey" />. |
==Medical Relevancy== | ==Medical Relevancy== | ||
Revision as of 01:22, 31 March 2017
Dimethylarginine Dimethylaminohydrolase
| |||||||||||
References
- ↑ 1.0 1.1 Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:17933965 doi:10.1152/ajpheart.00998.2007
- ↑ 2.0 2.1 2.2 Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:14664901 doi:10.1016/S1567-5688(03)00032-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:16698551 doi:10.1016/j.str.2006.03.006
- ↑ Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:23097221 doi:10.1002/path.4127
- ↑ Humm A, Fritsche E, Mann K, Göhl M, Huber R. Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue. Biochemical Journal. 1997 March 15;322(3):771-776. PMID:9148748 doi:10.1042/bj3220771
- ↑ 6.0 6.1 6.2 Rasheed M, Richter C, Chisty LT, Kirkpatrick J, Blackledge M, Webb MR, Driscoll PC. Ligand-dependent dynamics of the active site lid in bacterial Dimethyarginine Dimethylaminohydrolase. Biochemistry. 2014 Feb 18;53:1092-1104. PMCID:PMC3945819 doi:10.1021/bi4015924
- ↑ 7.0 7.1 Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of Dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006 May 2;45(17):5618-5630. PMID:16634643 doi:10.1021/bi052595m
- ↑ 8.0 8.1 Pace NJ, Weerpana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014 June;4(2):419-434. PMCID:4101490 doi:10.3390/biom4020419
- ↑ Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:10979862 doi:10.1016/S0165-6147(00)01499-1
- ↑ Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:14975444 doi:10.1016/j.freeradbiomed.2003.11.011
- ↑ Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:9883748
- ↑ Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:12461516 doi:10.1038/nrd960