We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1057
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
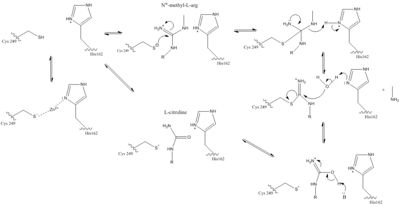
| - | Dimethylarginine Dimethyaminohydrolase (commonly known as DDAH) is a member of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Hydrolase hydrolase]</span> family of enzymes which use water to break down molecules <ref name="palm">Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/17933965 17933965]</span> doi:<span class="plainlinks">[http://ajpheart.physiology.org/content/293/6/H3227 10.1152/ajpheart.00998.2007]</span></ref>. Specifically, DDAH is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase nitric oxide synthase (NOS)]</span> regulator. Its metabolizes free arginine derivatives, namely <span class="plainlinks">[https://en.wikipedia.org/wiki/Asymmetric_dimethylarginine NѠ,NѠ-dimethyl-L-arginine (ADMA)]</span> and <span class="plainlinks">[https://en.wikipedia.org/wiki/Methylarginine NѠ-methyl-L-arginine (MMA)]</span> which competitively inhibit NOS <ref name="tran">Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14664901 14664901]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S1567568803000321 10.1016/S1567-5688(03)00032-1]</span></ref>. DDAH converts MMA and ADMA to <span class="plainlinks">[https://en.wikipedia.org/wiki/Citrulline L-citrulline]</span> and monoamine or dimethylamine <ref name="frey">Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16698551 16698551]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0969212606001717 10.1016/j.str.2006.03.006]</span></ref>. DDAH is expressed in the cytosol of cells in humans, mice, rates, sheep, cattle, and bacteria <ref name="palm" />. DDAH activity has been localized mainly to the brain, kidney, pancreas, and liver in these organisms. If DDAH is overexpressed, NOS can be activated <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase NOS (endothelial, neuronal, and inducible)]</span> <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. In humans, many diseases can come from improper control of NO levels including <span class="plainlinks">[https://en.wikipedia.org/wiki/Diabetes_mellitus diabetes mellitus]</span> and <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/high-blood-pressure/basics/definition/con-20019580 hypertension]</span>. Current research has identified several inhibitors of DDAH which could be important in fighting diseases involving irregular NO levels <ref name="frey" />. | + | Dimethylarginine Dimethyaminohydrolase <span class="plainlinks">[http://www.chem.qmul.ac.uk/iubmb/enzyme/EC3/5/3/18.html EC 3.5.3.18]</span> (commonly known as DDAH) is a member of the <span class="plainlinks">[https://en.wikipedia.org/wiki/Hydrolase hydrolase]</span> family of enzymes which use water to break down molecules <ref name="palm">Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/17933965 17933965]</span> doi:<span class="plainlinks">[http://ajpheart.physiology.org/content/293/6/H3227 10.1152/ajpheart.00998.2007]</span></ref>. Specifically, DDAH is a <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase nitric oxide synthase (NOS)]</span> regulator. Its metabolizes free arginine derivatives, namely <span class="plainlinks">[https://en.wikipedia.org/wiki/Asymmetric_dimethylarginine NѠ,NѠ-dimethyl-L-arginine (ADMA)]</span> and <span class="plainlinks">[https://en.wikipedia.org/wiki/Methylarginine NѠ-methyl-L-arginine (MMA)]</span> which competitively inhibit NOS <ref name="tran">Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14664901 14664901]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S1567568803000321 10.1016/S1567-5688(03)00032-1]</span></ref>. DDAH converts MMA and ADMA to <span class="plainlinks">[https://en.wikipedia.org/wiki/Citrulline L-citrulline]</span> and monoamine or dimethylamine <ref name="frey">Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/16698551 16698551]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0969212606001717 10.1016/j.str.2006.03.006]</span></ref>. DDAH is expressed in the cytosol of cells in humans, mice, rates, sheep, cattle, and bacteria <ref name="palm" />. DDAH activity has been localized mainly to the brain, kidney, pancreas, and liver in these organisms. If DDAH is overexpressed, NOS can be activated <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide_synthase NOS (endothelial, neuronal, and inducible)]</span> <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. In humans, many diseases can come from improper control of NO levels including <span class="plainlinks">[https://en.wikipedia.org/wiki/Diabetes_mellitus diabetes mellitus]</span> and <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/high-blood-pressure/basics/definition/con-20019580 hypertension]</span>. Current research has identified several inhibitors of DDAH which could be important in fighting diseases involving irregular NO levels <ref name="frey" />. |
==General Structure== | ==General Structure== | ||
| Line 41: | Line 41: | ||
== References == | == References == | ||
{{reflist}} | {{reflist}} | ||
| + | |||
| + | == Student Contributors == | ||
| + | *Natalie Van Ochten | ||
| + | *Kaitlyn Enderle | ||
| + | *Colton Junod | ||
| + | |||
| + | == 3D Structures of Dimethylarginine Dimethylaminohydrolase == | ||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci1 2CI1]</span> | ||
| + | |||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci3 2CI3]</span> | ||
| + | |||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci4 2CI4]</span> | ||
| + | |||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci5 2CI5]</span> | ||
| + | |||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci6 2CI6]</span> | ||
| + | |||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2ci7 2CI7]</span> | ||
| + | |||
| + | <span class="plainlinks">[http://proteopedia.org/wiki/index.php/2c6z 2C6Z]</span> | ||
Revision as of 03:22, 31 March 2017
Contents |
Dimethylarginine Dimethylaminohydrolase
| |||||||||||
References
- ↑ 1.0 1.1 Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:17933965 doi:10.1152/ajpheart.00998.2007
- ↑ 2.0 2.1 2.2 Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:14664901 doi:10.1016/S1567-5688(03)00032-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:16698551 doi:10.1016/j.str.2006.03.006
- ↑ Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:23097221 doi:10.1002/path.4127
- ↑ Humm A, Fritsche E, Mann K, Göhl M, Huber R. Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue. Biochemical Journal. 1997 March 15;322(3):771-776. PMID:9148748 doi:10.1042/bj3220771
- ↑ 6.0 6.1 6.2 Rasheed M, Richter C, Chisty LT, Kirkpatrick J, Blackledge M, Webb MR, Driscoll PC. Ligand-dependent dynamics of the active site lid in bacterial Dimethyarginine Dimethylaminohydrolase. Biochemistry. 2014 Feb 18;53:1092-1104. PMCID:PMC3945819 doi:10.1021/bi4015924
- ↑ 7.0 7.1 Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of Dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006 May 2;45(17):5618-5630. PMID:16634643 doi:10.1021/bi052595m
- ↑ 8.0 8.1 Pace NJ, Weerpana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014 June;4(2):419-434. PMCID:4101490 doi:10.3390/biom4020419
- ↑ Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:10979862 doi:10.1016/S0165-6147(00)01499-1
- ↑ Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:14975444 doi:10.1016/j.freeradbiomed.2003.11.011
- ↑ Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:9883748
- ↑ Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:12461516 doi:10.1038/nrd960
Student Contributors
- Natalie Van Ochten
- Kaitlyn Enderle
- Colton Junod