Sandbox Reserved 1069
From Proteopedia
| Line 23: | Line 23: | ||
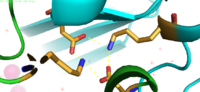
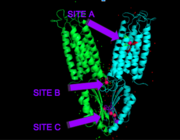
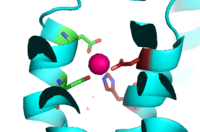
Binding site A is in the center of the transmembrane domain, attached and confined via residues from the TM2 and TM5 helices. The TM2 domain has <scene name='69/694236/Asp45/1'>Asp45</scene> and Asp49, and the TM5 has His153 and Asp157. The TM5 helix is significantly shorter than the other 5 helices around it, and this length forms a cavity in the membrane. In turn, this cavity is able to bind a Zn<sup>2+</sup> ion. This site is the protein's active site, meaning that this is where the zinc is able to attach and eventually exit the cell via proton transport. This particular site has an ideal tetrahedron among its residues which is preferred for Zn<sup>2+</sup>, thus making it the perfect active site for Zn<sup>2+</sup> to bind. | Binding site A is in the center of the transmembrane domain, attached and confined via residues from the TM2 and TM5 helices. The TM2 domain has <scene name='69/694236/Asp45/1'>Asp45</scene> and Asp49, and the TM5 has His153 and Asp157. The TM5 helix is significantly shorter than the other 5 helices around it, and this length forms a cavity in the membrane. In turn, this cavity is able to bind a Zn<sup>2+</sup> ion. This site is the protein's active site, meaning that this is where the zinc is able to attach and eventually exit the cell via proton transport. This particular site has an ideal tetrahedron among its residues which is preferred for Zn<sup>2+</sup>, thus making it the perfect active site for Zn<sup>2+</sup> to bind. | ||
| - | [[Image:Binding_site_A.fw.png|thumb|Binding Site A showing TM2 domain (left) and TM5 domain (right). The Asp45 and Asp49 as well as the His153 and Asp157 are the coordination residues in the acitve site | + | [[Image:Binding_site_A.fw.png|200px|right|thumb|Binding Site A showing TM2 domain (left) and TM5 domain (right). The Asp45 and Asp49 as well as the His153 and Asp157 are the coordination residues in the acitve site]][ |
It is important to note that the structure of this binding site is rigid because of the coordination of the Zn<sup>2+</sup> between the four residues. This rigidity is indicative that any slight shift on either of the helices will cause a drastic readjustment of the coordination of Zn<sup>2+</sup>. In addition, there are no outer-shell constraints to hold the residues in place, which means that with a readjustment of the molecule, there is no energy being expended to bind or release another Zn<sup>2+</sup> molecule. Therefore, the Zn<sup>2+</sup> is able to rapidly release and a new Zn<sup>2+</sup> can bind again with a simple reorientation or shift of the molecule. This rapid on off bind and release mechanism is the regulator of homeostatic levels of Zn<sup>2+</sup> in the cell. This regulation is so rapid in fact, it is significantly faster than other Zn<sup>2+</sup> exchange rate proteins- by several orders of magnitude. | It is important to note that the structure of this binding site is rigid because of the coordination of the Zn<sup>2+</sup> between the four residues. This rigidity is indicative that any slight shift on either of the helices will cause a drastic readjustment of the coordination of Zn<sup>2+</sup>. In addition, there are no outer-shell constraints to hold the residues in place, which means that with a readjustment of the molecule, there is no energy being expended to bind or release another Zn<sup>2+</sup> molecule. Therefore, the Zn<sup>2+</sup> is able to rapidly release and a new Zn<sup>2+</sup> can bind again with a simple reorientation or shift of the molecule. This rapid on off bind and release mechanism is the regulator of homeostatic levels of Zn<sup>2+</sup> in the cell. This regulation is so rapid in fact, it is significantly faster than other Zn<sup>2+</sup> exchange rate proteins- by several orders of magnitude. | ||
Revision as of 16:05, 31 March 2017
Introduction
Zinc transporter is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological real, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which is biologically relevant because while these metals are necessary for different biological functions, they can prove fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researcher's better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||