Sandbox Reserved 1069
From Proteopedia
| Line 8: | Line 8: | ||
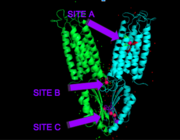
YiiP is a homodimer [https://en.wikipedia.org/wiki/Protein_dimer (protein dimer)], with each monomer consisting of 238 residues with a TransMembrane (<scene name='69/694236/Tmd/1'>TMD</scene>) and C-Terminal (<scene name='69/694236/Ctd/1'>CTD</scene>) domain that are connected via a charge interlocking mechanism located on a flexible loop. There are three Zn<sup>2+</sup> binding sites present. Site A is located in both TMDs of the protein, site C is located in the CTD, and site B is located at the junction of the domains join. The TMD, where Zn<sup>2+</sup> binding site A resides, consists of 6 transmembrane (TM) <scene name='75/756372/Sixhelices/1'>helices</scene>, 4 of which (TM1, TM2, TM4, TM5) pivot about the ion binding site A. The remaining two helices, TM3 and TM6, are oriented <scene name='75/756372/2antiparallel/1'>antiparallel</scene> to the bundle. Movement of these helices play a role in the function of Zn<sup>2+</sup> transport. | YiiP is a homodimer [https://en.wikipedia.org/wiki/Protein_dimer (protein dimer)], with each monomer consisting of 238 residues with a TransMembrane (<scene name='69/694236/Tmd/1'>TMD</scene>) and C-Terminal (<scene name='69/694236/Ctd/1'>CTD</scene>) domain that are connected via a charge interlocking mechanism located on a flexible loop. There are three Zn<sup>2+</sup> binding sites present. Site A is located in both TMDs of the protein, site C is located in the CTD, and site B is located at the junction of the domains join. The TMD, where Zn<sup>2+</sup> binding site A resides, consists of 6 transmembrane (TM) <scene name='75/756372/Sixhelices/1'>helices</scene>, 4 of which (TM1, TM2, TM4, TM5) pivot about the ion binding site A. The remaining two helices, TM3 and TM6, are oriented <scene name='75/756372/2antiparallel/1'>antiparallel</scene> to the bundle. Movement of these helices play a role in the function of Zn<sup>2+</sup> transport. | ||
| - | A large portion of the protein containing binding site C, the CTD, approximately 30 Å in length<sup>[https://www.bnl.gov/isd/documents/71335.pdf]</sup>, protrudes into the cytoplasm functioning as a | + | A large portion of the protein containing binding site C, the CTD, approximately 30 Å in length<sup>[https://www.bnl.gov/isd/documents/71335.pdf]</sup>, protrudes into the cytoplasm functioning as a Zn<sup>2+</sup> sensor within the cell. Zn<sup>2+</sup> binding at site C helps hold the CTD together and is thought to stabilize conformational changes in YiiP. YiiP has two different functional conformations which dictates whether or not YiiP is open to the periplasm or the cytoplasm. An interlocked salt bridge connects the two domains with the Lys77 and the Asp207 from each monomer. This [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridge] acts as the hinge for Yiip's conformational changes. |
===Interlocking Salt Bridge=== | ===Interlocking Salt Bridge=== | ||
| - | The <scene name='75/756372/Saltbridgelabel/1'>salt bridge</scene> formation between Lys77 and Asp207 of each domain of YiiP form an interlocking interaction that acts as the pivot point of the conformational change that drives the function of YiiP. The salt bridge is disrupted when | + | The <scene name='75/756372/Saltbridgelabel/1'>salt bridge</scene> formation between Lys77 and Asp207 of each domain of YiiP form an interlocking interaction that acts as the pivot point of the conformational change that drives the function of YiiP. The salt bridge is disrupted when Zn<sup>2+</sup> is bound, due to movement of the antiparallel helices. This disrupts the ion-ion attractions that established the salt bridge- causing a conformational shift in YiiP. This salt bridge also aids in holding the two monomers together. [[Image:Saltbridge.png|200px|left|thumb|Lys77 and Asp207 Salt Bridges]]<scene name='75/756372/Hydrophobic1/1'>Hydrophobic</scene> residues around the salt bridge further stabilize the two domains in the v-shaped void where the domains connect. This prevents degradation of the protein's interlock via interactions with the environment. |
=== Zn<sup>2+</sup> Binding Sites === | === Zn<sup>2+</sup> Binding Sites === | ||
| Line 20: | Line 20: | ||
'''Binding Site A''' | '''Binding Site A''' | ||
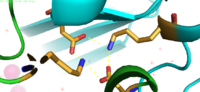
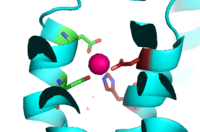
| - | Binding site A is in the center of the transmembrane domain, attached and confined via residues from the TM2 and TM5 helices. The TM2 domain has <scene name='69/694236/Asp45/1'>Asp45</scene> and Asp49, and the TM5 has His153 and Asp157, which facilitate the binding and releasing of Zn<sup>2+</sup> within the sites. The TM5 helix is significantly shorter than the other 5 helices around it, and this length forms a cavity in the membrane. In turn, this cavity is able to bind a Zn<sup>2+</sup> ion. This site is the protein's active site, meaning that this is where the | + | Binding site A is in the center of the transmembrane domain, attached and confined via residues from the TM2 and TM5 helices. The TM2 domain has <scene name='69/694236/Asp45/1'>Asp45</scene> and Asp49, and the TM5 has His153 and Asp157, which facilitate the binding and releasing of Zn<sup>2+</sup> within the sites. The TM5 helix is significantly shorter than the other 5 helices around it, and this length forms a cavity in the membrane. In turn, this cavity is able to bind a Zn<sup>2+</sup> ion. This site is the protein's active site, meaning that this is where the Zn<sup>2+</sup> is able to attach and eventually exit the cell via proton transport. This particular site has an ideal tetrahedron among its residues which is preferred for Zn<sup>2+</sup>, thus making it the perfect active site for Zn<sup>2+</sup> to bind. |
[[Image:Binding_site_A.fw.png|200px|left|thumb|Binding Site A showing TM2 domain (left) and TM5 domain (right). The Asp45 and Asp49 as well as the His153 and Asp157 are the coordination residues in the acitve site]] | [[Image:Binding_site_A.fw.png|200px|left|thumb|Binding Site A showing TM2 domain (left) and TM5 domain (right). The Asp45 and Asp49 as well as the His153 and Asp157 are the coordination residues in the acitve site]] | ||
Revision as of 17:01, 31 March 2017
Introduction
Zinc transporter is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological real, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which is biologically relevant because while these metals are necessary for different biological functions, they can prove fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researcher's better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||