User:Luke Edward Severinac/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
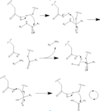
Caspase 6 is a part of the cystine aspartic protease family that cleaves proteins at the TEVD sequence. in its monomeric form with protein ligand bound, its catalytic residues are <scene name='75/752344/His121/1'>Histidine 121</scene>, <scene name='75/752344/Glu123/1'>Glutamate 123</scene>, and <scene name='75/752344/Cys163/1'>Cystine 163</scene>.[[Image:Mechanism caspase 6.PNG|100 px|left|thumb|Cystine Aspartase mechanism]] Together, these residues form a <scene name='75/752344/Caspase-6_catalytic_triad_real/1'>catalytic triad</scene> that cleaves a <scene name='75/752344/Protein_ligand/1'>protein ligand</scene>. | Caspase 6 is a part of the cystine aspartic protease family that cleaves proteins at the TEVD sequence. in its monomeric form with protein ligand bound, its catalytic residues are <scene name='75/752344/His121/1'>Histidine 121</scene>, <scene name='75/752344/Glu123/1'>Glutamate 123</scene>, and <scene name='75/752344/Cys163/1'>Cystine 163</scene>.[[Image:Mechanism caspase 6.PNG|100 px|left|thumb|Cystine Aspartase mechanism]] Together, these residues form a <scene name='75/752344/Caspase-6_catalytic_triad_real/1'>catalytic triad</scene> that cleaves a <scene name='75/752344/Protein_ligand/1'>protein ligand</scene>. | ||
===Zinc Exosite=== | ===Zinc Exosite=== | ||
| + | Caspase-6 function is inhibited by the binding of a zinc ion, which binds to an allosteric site instead of the active site. This allosteric site is located on the outside of the protein and it is distal to the active site. The Zinc ion is bound to three amino acid residues, Lysine-36, Glutamate-244, and Histidine-287, once the ion is bound to the protein it is then stabalized by a single water molecule. The binding at the exosite is suggested to cause a conformational change to the protein, which then cause a change in the active site, which inhibits Caspase-6's ability to bind to substrate. | ||
=='''Activation of Caspase-6'''== | =='''Activation of Caspase-6'''== | ||
| - | + | Before Caspase-6 is a functional and active dimer, the enzyme exists as a procaspase, also know as a zymogen. This precursor enzyme is modified by self-processing, a characteristic unique to Caspase-6. The unprocessed enzyme contains a small and large subunit, a pro domain, as well as a intersubunit linker. To become active, the intersubunit linker binds to the active site, where it is then cleaved. Other cleavages must occur as well for the enzyme to become active, specifically at TETD23 of the pro-domain, DVVD179, and TEVD193 amino acid sequences. Cleavage at these sites occurs in a specific sequence. To begin, the site within the pro-domain, TETD23, must be cleaved. This cleavage is then followed by either DVVD179 or TEVD193. It has been proposed that this sequence of cleavage is due to the structure of Caspase-6, which allows the pro-domain to be more readily available to enter the active site. To some extent, the pro-domain inhibits Caspase-6's ability to cleave the intersubunit loop and self-activate, but this also happens in a currently unknown mechanism. The pro-domain is released after the cleavage at TETD23, which then allows the two subunits to interact to form the active dimer. | |
| - | Before Caspase-6 is a functional and active dimer, the enzyme exists as a procaspase. This precursor enzyme is modified by self-processing, a characteristic unique to Caspase-6. The unprocessed enzyme contains a small and large subunit, a pro domain, as well as a intersubunit linker. To become active, the intersubunit linker binds to the active site, where it is then cleaved. Other cleavages must occur as well for the enzyme to become active, specifically at TETD23 of the pro-domain, DVVD179, and TEVD193 amino acid sequences. Cleavage at these sites occurs in a specific sequence. To begin, the site within the pro-domain, TETD23, must be cleaved. This cleavage is then followed by either DVVD179 or TEVD193. It has been proposed that this sequence of cleavage is due to the structure of Caspase-6, which allows the pro-domain to be more readily available to enter the active site. To some extent, the pro-domain inhibits Caspase-6's ability to cleave the intersubunit loop and self-activate, but this also happens in a currently unknown mechanism. The pro-domain is released after the cleavage at TETD23, which then allows the two subunits to interact to form the active dimer. | + | |
Caspase-6 can also be activated by other caspases as an alternate to auto-activation. | Caspase-6 can also be activated by other caspases as an alternate to auto-activation. | ||
| Line 45: | Line 45: | ||
<scene name='75/752344/Uncleaved_caspase_6/1'>inactive caspase</scene> | <scene name='75/752344/Uncleaved_caspase_6/1'>inactive caspase</scene> | ||
| - | [[Image:Binding grove active caspase 6.png]] | ||
== Relevance == | == Relevance == | ||
Revision as of 22:22, 2 April 2017
Caspase-6 in Homo sapiens
| |||||||||||
References
Wang, Xiao-Jun, Qin Cao, Yan Zhang, and Xiao-Dong Su. "Activation and Regulation of Caspase-6 and Its Role in Neurodegenerative Diseases." Annual Review of Pharmacology and Toxicology 55.1 (2015): 553-72. Web.
Wang XJ, Cao Q, Liu X, Wang KT, Mi W, et al. 2010. Crystal structures of human caspase 6 reveal a new mechanism for intramolecular cleavage self-activation. EMBO Rep. 11: 841–47
(self cleavage article)
http://www.rcsb.org/pdb/explore/explore.do?structureId=2WDP (this is the non-self cleaved protien)