User:Luke Edward Severinac/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
[[Image:4FXO.PNG|100 px|left|thumb|This is the figure legend of the thumbnail]] | [[Image:4FXO.PNG|100 px|left|thumb|This is the figure legend of the thumbnail]] | ||
| - | ==''' | + | =='''Structure'''== |
===Active Site=== | ===Active Site=== | ||
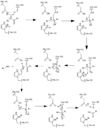
In order to function as an endoprotease, Caspase-6 binds a <scene name='75/752344/Protein_ligand_real/1'>ligand</scene>, which can include neuronal proteins and tubulins [https://en.wikipedia.org/wiki/Tubulin], in its active site. This binding groove contains three critical amino acid residues necessary to perform cleavage of the peptide bonds. Together,<scene name='75/752344/His121_real/1'>His-121</scene>, <scene name='75/752344/Glu123_real/1'>Glu-123</scene>, and <scene name='75/752344/Cys163_real/1'>Cys-163</scene> form a <scene name='75/752344/Catalytic_triad_real/1'>catalytic triad</scene>[[Image:The real caspase mechanism.jpg|100 px|left|thumb|Cystine Aspartase mechanism]]. In the theorized mechanism, His-121 acts as an acid catalyst, Glu-123 acts as a base catalyst to deprotonate Cys-163, which then acts as covalent catalyst. | In order to function as an endoprotease, Caspase-6 binds a <scene name='75/752344/Protein_ligand_real/1'>ligand</scene>, which can include neuronal proteins and tubulins [https://en.wikipedia.org/wiki/Tubulin], in its active site. This binding groove contains three critical amino acid residues necessary to perform cleavage of the peptide bonds. Together,<scene name='75/752344/His121_real/1'>His-121</scene>, <scene name='75/752344/Glu123_real/1'>Glu-123</scene>, and <scene name='75/752344/Cys163_real/1'>Cys-163</scene> form a <scene name='75/752344/Catalytic_triad_real/1'>catalytic triad</scene>[[Image:The real caspase mechanism.jpg|100 px|left|thumb|Cystine Aspartase mechanism]]. In the theorized mechanism, His-121 acts as an acid catalyst, Glu-123 acts as a base catalyst to deprotonate Cys-163, which then acts as covalent catalyst. | ||
===Zinc Exosite=== | ===Zinc Exosite=== | ||
| - | Caspase-6 function is inhibited by the binding of a <scene name='75/752344/Zinc_caspase-6/1'>Zinc</scene> ion, which binds to an <scene name='75/752344/Caspase6_allosteric_site/1'>allosteric site</scene> instead of the <scene name='75/752344/Caspase6_allostericactiv_site/1'>active site</scene>. This allosteric site is located on the outside of the protein and it is distal to the active site. The Zinc ion is bound to three | + | Caspase-6 function is inhibited by the binding of a <scene name='75/752344/Zinc_caspase-6/1'>Zinc</scene> ion, which binds to an <scene name='75/752344/Caspase6_allosteric_site/1'>allosteric site</scene> instead of the <scene name='75/752344/Caspase6_allostericactiv_site/1'>active site</scene>. This allosteric site is located on the outside of the protein and it is distal to the active site. The Zinc ion is bound to <scene name='75/752344/Caspase6_allostericactiv_site/1'>three residues</scene>, Lys-36, Glu-244, and His-287, once the ion is bound to the protein it is then stabilized by a <scene name='75/752344/H20_zinc_binding_casp/1'>water molecule</scene>. The binding of Zinc at the exosite is suggested to cause a conformational change to the protein, which then causes a change in the active site, which inhibits Caspase-6's ability to bind to substrate. Zinc binding to the exosite is tightly regulated, because it inhibits Caspase-6's ability to inititate apoptosis. |
=='''Activation of Caspase-6'''== | =='''Activation of Caspase-6'''== | ||
Revision as of 03:15, 3 April 2017
Caspase-6 in Homo sapiens
| |||||||||||
References
- ↑ Wang XJ, Cao Q, Zhang Y, Su XD. Activation and regulation of caspase-6 and its role in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:553-72. doi:, 10.1146/annurev-pharmtox-010814-124414. Epub 2014 Oct 17. PMID:25340928 doi:http://dx.doi.org/10.1146/annurev-pharmtox-010814-124414
- ↑ 2.0 2.1 Velazquez-Delgado EM, Hardy JA. Zinc-Mediated Allosteric Inhibition of Caspase-6. J Biol Chem. 2012 Aug 13. PMID:22891250 doi:http://dx.doi.org/10.1074/jbc.M112.397752
Wang, Xiao-Jun, Qin Cao, Yan Zhang, and Xiao-Dong Su. "Activation and Regulation of Caspase-6 and Its Role in Neurodegenerative Diseases." Annual Review of Pharmacology and Toxicology 55.1 (2015): 553-72. Web.
Wang XJ, Cao Q, Liu X, Wang KT, Mi W, et al. 2010. Crystal structures of human caspase 6 reveal a new mechanism for intramolecular cleavage self-activation. EMBO Rep. 11: 841–47
(self cleavage article)
http://www.rcsb.org/pdb/explore/explore.do?structureId=2WDP (this is the non-self cleaved protien)