User:Luke Edward Severinac/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
[[Image:4FXO.PNG|100 px|left|thumb|This is the figure legend of the thumbnail]] | [[Image:4FXO.PNG|100 px|left|thumb|This is the figure legend of the thumbnail]] | ||
| - | ==''' | + | =='''Structure'''== |
| + | ===Sequence=== | ||
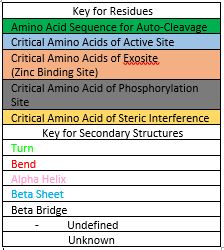
The image below describes the amino acid sequence of Caspase-6, highlighting critical amino acids and sequences necessary for function. It also highlights the secondary structures, which make up the folded protein, and sequences which become cleaved during zymogen processing. | The image below describes the amino acid sequence of Caspase-6, highlighting critical amino acids and sequences necessary for function. It also highlights the secondary structures, which make up the folded protein, and sequences which become cleaved during zymogen processing. | ||
| Line 21: | Line 22: | ||
=='''Activation of Caspase-6'''== | =='''Activation of Caspase-6'''== | ||
Before Caspase-6 is a functional and active dimer, the enzyme exists as a <scene name='75/752344/Caspase-6_zymogen/1'>procaspase</scene>, also known as zymogen [https://en.wikipedia.org/wiki/Zymogen]. Caspase-6 can be activated by acting as a substrate for other caspases, particularly Caspase-3, as well as other enzymes. It becomes cleaved by these enzymes and proceeds to its <scene name='75/752344/Active_caspase_6/1'>active dimer conformation</scene>. It was observed that Caspase-6 became active without alternate enzymes present, which suggested that Caspase-6 utilizes a self-cleavage mechanism. Now, self-processing, a characteristic unique to Caspase-6, is recognized as the primary mechanism for Caspase-6 activation. The unprocessed enzyme contains a <scene name='75/752344/Caspase-6_small_subunit/1'>small</scene> and <scene name='75/752344/Caspase-6_large_real/1'>large</scene> subunit, a <scene name='75/752344/Caspase-6_pro-domain/1'>pro-domain</scene>, as well as an intersubunit linker. To become active, the intersubunit linker binds to the active site, where it is then cleaved. Other cleavages must occur as well for the enzyme to become active, specifically at TETD23 (these residues are not visible in the crystallized structure) of the pro-domain, <scene name='75/752344/Caspase-6_176-179_cleavage/1'>DVVD179</scene>, and <scene name='75/752344/Caspase-6_tevd193/1'>TEVD193</scene> amino acid sequences. Cleavage at these sites occurs in a <scene name='75/752344/Caspase-6_cleavage_sites/1'>specific sequence</scene>. First, the site within the pro-domain, TETD23, must be cleaved. This cleavage is then followed by either DVVD179 or TEVD193. | Before Caspase-6 is a functional and active dimer, the enzyme exists as a <scene name='75/752344/Caspase-6_zymogen/1'>procaspase</scene>, also known as zymogen [https://en.wikipedia.org/wiki/Zymogen]. Caspase-6 can be activated by acting as a substrate for other caspases, particularly Caspase-3, as well as other enzymes. It becomes cleaved by these enzymes and proceeds to its <scene name='75/752344/Active_caspase_6/1'>active dimer conformation</scene>. It was observed that Caspase-6 became active without alternate enzymes present, which suggested that Caspase-6 utilizes a self-cleavage mechanism. Now, self-processing, a characteristic unique to Caspase-6, is recognized as the primary mechanism for Caspase-6 activation. The unprocessed enzyme contains a <scene name='75/752344/Caspase-6_small_subunit/1'>small</scene> and <scene name='75/752344/Caspase-6_large_real/1'>large</scene> subunit, a <scene name='75/752344/Caspase-6_pro-domain/1'>pro-domain</scene>, as well as an intersubunit linker. To become active, the intersubunit linker binds to the active site, where it is then cleaved. Other cleavages must occur as well for the enzyme to become active, specifically at TETD23 (these residues are not visible in the crystallized structure) of the pro-domain, <scene name='75/752344/Caspase-6_176-179_cleavage/1'>DVVD179</scene>, and <scene name='75/752344/Caspase-6_tevd193/1'>TEVD193</scene> amino acid sequences. Cleavage at these sites occurs in a <scene name='75/752344/Caspase-6_cleavage_sites/1'>specific sequence</scene>. First, the site within the pro-domain, TETD23, must be cleaved. This cleavage is then followed by either DVVD179 or TEVD193. | ||
| - | Despite the sequence similarities between TETD23 and TEVD193 cleavage sites, the TETD23 cleavage site is always cleaved before TEVD193. It has been proposed that this sequence of cleavage is due to the <scene name='75/752344/Caspase-6_pr-domai-active-site/1'>structure of Caspase-6's zymogen</scene>, which allows the pro-domain to be more readily available to enter the active site. To some extent, the pro-domain inhibits Caspase-6's ability to cleave the intersubunit loop and self-activate, but this happens in a currently unknown mechanism. The result of the TETD23 cleavage site priority is that the prodomain acts as a “suicide protector”, which protects the TEVD193 cleavage site from self-cleavage | + | Despite the sequence similarities between TETD23 and TEVD193 cleavage sites, the TETD23 cleavage site is always cleaved before TEVD193. It has been proposed that this sequence of cleavage is due to the <scene name='75/752344/Caspase-6_pr-domai-active-site/1'>structure of Caspase-6's zymogen</scene>, which allows the pro-domain to be more readily available to enter the active site. To some extent, the pro-domain inhibits Caspase-6's ability to cleave the intersubunit loop and self-activate, but this happens in a currently unknown mechanism. The result of the TETD23 cleavage site priority is that the prodomain acts as a “suicide protector”, which protects the TEVD193 cleavage site from self-cleavage<ref name="CryStructureNewMechForSelfAct">PMID: 20890311 </ref>. This protection is necessary when there are low levels of inactive proteins, which must be preserved, in the tissue. The pro-domain is released after the cleavage at TETD23 and cleavage of the intersubunit links follow. This then allows the two subunits to interact to form the active dimer. The intramolecular cleavage of TEVD193 is essential for the initiation caspase-6 activation without other caspases present. |
| Line 32: | Line 33: | ||
| - | ==''' | + | =='''Medical Relevance'''== |
===Caspase-6 involvement in Alzheimer's Disease=== | ===Caspase-6 involvement in Alzheimer's Disease=== | ||
| - | Caspase-6 activity is associated with the formation of lesions within the Alzheimer's Disease (AD) | + | Caspase-6 is known to be involved in many neurodegenerative diseases, one of which is Alzheimer's disease. Caspase-6 activity is associated with the formation of lesions within the Alzheimer's Disease (AD)[http://www.alz.org/].Lesions can be found in early stages of AD<ref name="ActiveRegofCasp6andNDdisease">PMID: 25340928 </ref>. A proapoptotic protein, p53, is present at increased levels within AD brains, which seems to directly increase the transcription of Caspase-6, which indirectly influences apoptosis of neurons. Future treatments of AD include selective inhibition of active Caspase-6 proteins; staining has found active Caspase-6 within the hippocampus and cortex of the brain within a varying severity of AD cases. This suggests that Caspase-6 plays a predominate role in the pathophysiology of AD. There has been research conducted that shows activation of Caspase-6 in AD could cause disruption of the cytoskeleton network of neurons and lead to neuronal apoptosis<ref name="ActiveRegofCasp6andNDdisease">PMID: 25340928 </ref>. |
Revision as of 01:27, 4 April 2017
Caspase-6 in Homo sapiens
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Wang XJ, Cao Q, Zhang Y, Su XD. Activation and regulation of caspase-6 and its role in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:553-72. doi:, 10.1146/annurev-pharmtox-010814-124414. Epub 2014 Oct 17. PMID:25340928 doi:http://dx.doi.org/10.1146/annurev-pharmtox-010814-124414
- ↑ 2.0 2.1 Velazquez-Delgado EM, Hardy JA. Zinc-Mediated Allosteric Inhibition of Caspase-6. J Biol Chem. 2012 Aug 13. PMID:22891250 doi:http://dx.doi.org/10.1074/jbc.M112.397752
- ↑ Wang XJ, Cao Q, Liu X, Wang KT, Mi W, Zhang Y, Li LF, Leblanc AC, Su XD. Crystal structures of human caspase 6 reveal a new mechanism for intramolecular cleavage self-activation. EMBO Rep. 2010 Oct 1. PMID:20890311 doi:10.1038/embor.2010.141
- ↑ Velazquez-Delgado EM, Hardy JA. Phosphorylation regulates assembly of the caspase-6 substrate-binding groove. Structure. 2012 Apr 4;20(4):742-51. Epub 2012 Apr 3. PMID:22483120 doi:10.1016/j.str.2012.02.003