We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
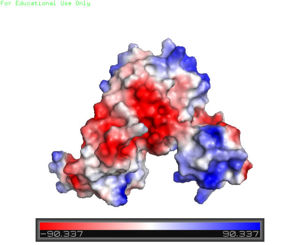
The AdcR MarR transcriptional regulator's structure resembles the other proteins in the same family as mentioned before; however, the most notable differences are found in the winged helix-turn-helix (wHTH) motif that assists in binding DNA. The major groove of DNA is bound to the recognition helix while the wings grip onto the minor grooves of DNA <ref>PMID:22085181</ref>. The charge map on the right highlights the <font color='blue'>positively</font> charged areas, which stabilize the negatively charged backbone of DNA. Although AdcR is a highly alpha helical protein, the "wings" of the DNA binding domain consist of two anti parallel beta strands that are made up of several positively charged residues. There is a wHTH domain on each monomer of the protein. | The AdcR MarR transcriptional regulator's structure resembles the other proteins in the same family as mentioned before; however, the most notable differences are found in the winged helix-turn-helix (wHTH) motif that assists in binding DNA. The major groove of DNA is bound to the recognition helix while the wings grip onto the minor grooves of DNA <ref>PMID:22085181</ref>. The charge map on the right highlights the <font color='blue'>positively</font> charged areas, which stabilize the negatively charged backbone of DNA. Although AdcR is a highly alpha helical protein, the "wings" of the DNA binding domain consist of two anti parallel beta strands that are made up of several positively charged residues. There is a wHTH domain on each monomer of the protein. | ||
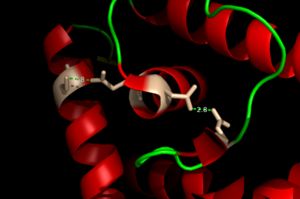
| - | The <scene name='69/694230/Whth_2/1'>winged helix-turn-helix</scene> domain is made up of the <font color=' | + | The <scene name='69/694230/Whth_2/1'>winged helix-turn-helix</scene> domain is made up of the <font color='blue'>alpha 2</font> and <font color='blue'>alpha 4 helices</font> along with <font color='blue'>anti-parallel beta sheets</font> on each side. Only one monomer is shown. The recognition helix, or the alpha 4 helix, binds the major groove of DNA through hydrogen bonding and Van der Waals interactions between exposed bases. The wings of the helix bind the minor groove of DNA while the other helices stabilize the DNA and Protein upon binding. The two anti parallel beta sheets contain several <scene name='69/694230/Positive_residues_on_wing/1'>Arginine, Asparagine, and Lysine residues</scene> that stabilize this interaction between DNA. |
=== Hydrogen Bond Network === | === Hydrogen Bond Network === | ||
Revision as of 18:45, 14 April 2017
Adhesin Competence Regulator
| |||||||||||
References
- ↑ Sanson M, Makthal N, Flores AR, Olsen RJ, Musser JM, Kumaraswami M. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res. 2015 Jan;43(1):418-32. doi: 10.1093/nar/gku1304. Epub 2014 Dec, 15. PMID:25510500 doi:http://dx.doi.org/10.1093/nar/gku1304
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Fraústo da Silva J, Williams R. The Biological Chemistry of Elements: The Inorganic Chemistry of Life. Second ed. Oxford University Press; Oxford: 2001.
- ↑ Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009 Oct;109(10):4644-81. doi: 10.1021/cr900077w. PMID:19788177 doi:http://dx.doi.org/10.1021/cr900077w
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, Giedroc DP. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010, Sep 8. PMID:20804771 doi:http://dx.doi.org/10.1016/j.jmb.2010.08.030
- ↑ Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, Giedroc DP. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010, Sep 8. PMID:20804771 doi:http://dx.doi.org/10.1016/j.jmb.2010.08.030
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532