We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
===Introduction=== | ===Introduction=== | ||
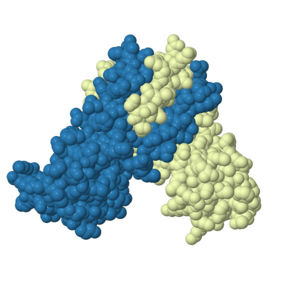
[[Image:3TGNspacefill.jpg | 300px | right|thumb|3TGN space fill model. Blue and white representing symmetric monomers.]] | [[Image:3TGNspacefill.jpg | 300px | right|thumb|3TGN space fill model. Blue and white representing symmetric monomers.]] | ||
| - | Adhesin Competence Regulator (AdcR) is a transcriptional regulator that controls the activation of over seventy genes within the bacteria [https://en.wikipedia.org/wiki/Streptococcus_pneumoniae''Streptococcus pneumoniae'']<ref>DOI:10.1093/nar/gku1304 </ref>. | + | Adhesin Competence Regulator (AdcR) is a transcriptional regulator that controls the activation of over seventy genes within the bacteria [https://en.wikipedia.org/wiki/Streptococcus_pneumoniae''Streptococcus pneumoniae''] and is a member of the multiple antibiotic resistance regulator (MarR) protein family <ref>DOI:10.1093/nar/gku1304 </ref>. Members of the MarR protein family conserve a number of features including a general triangular shape, a two fold pseudosymmetric homo dimer, and a wingled helix-turn-helix pattern [https://en.wikipedia.org/wiki/Helix-turn-helix (wHTH)]. Consistent with AdcR's identity as a member of the MarR protein family, AdcR exhibits these conserved features. This structure calls for multiple zinc binding sites that facilitate protein conformational change allowing for DNA binding and regulation through the wHTH domain. |
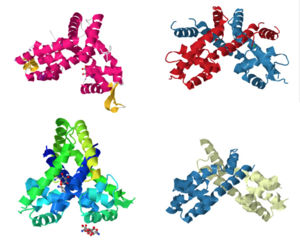
| - | [[Image:Image1.jpg | 300 px|left|thumb|Proteins 3BPX, 2FBK, 3KP5, and 2PFB (members of the MarR family) are pictured above.]] | + | |
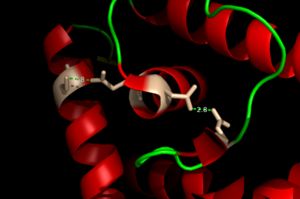
| - | + | Zinc plays a vital role in organism homeostasis acting as a [https://en.wikipedia.org/wiki/Cofactor_(biochemistry) co-factor] and a regulator of enzymatic activity, however zinc can lead to cell toxicity and deficiency of other vital metals that are also necessary for protein function <ref> Fraústo da Silva J, Williams R. The Biological Chemistry of Elements: The Inorganic Chemistry of Life. Second ed. Oxford University Press; Oxford: 2001.</ref><ref> DOI: 10.1021/cr900077w</ref>. The importance of AdcR in ''Streptococcus pneumoniae'' can be understood provided its ability to regulate zinc transfer proteins within the bacteria. Contrasting with other members of the MarR family, AdcR is metal dependent. Binding of Zinc allows AdcR to bind DNA and activate the transcription of high-affinity Zinc specific uptake transporters. The binding of Zinc induces a conformational change that allows for a hydrogen bond network between helices of the binding domain. It is believed that this hydrogen bond network is the allosteric activator needed to expose residues that bind the bases along the major groove of the DNA <ref>PMID:22085181</ref>. | |
| + | [[Image:Image1.jpg |300 px|left|thumb|Proteins 3BPX, 2FBK, 3KP5, and 2PFB (members of the MarR family) are pictured above.]] | ||
| + | |||
== '''DNA Binding''' == | == '''DNA Binding''' == | ||
Revision as of 23:47, 17 April 2017
Adhesin Competence Regulator
| |||||||||||
References
- ↑ Sanson M, Makthal N, Flores AR, Olsen RJ, Musser JM, Kumaraswami M. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res. 2015 Jan;43(1):418-32. doi: 10.1093/nar/gku1304. Epub 2014 Dec, 15. PMID:25510500 doi:http://dx.doi.org/10.1093/nar/gku1304
- ↑ Fraústo da Silva J, Williams R. The Biological Chemistry of Elements: The Inorganic Chemistry of Life. Second ed. Oxford University Press; Oxford: 2001.
- ↑ Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009 Oct;109(10):4644-81. doi: 10.1021/cr900077w. PMID:19788177 doi:http://dx.doi.org/10.1021/cr900077w
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, Giedroc DP. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010, Sep 8. PMID:20804771 doi:http://dx.doi.org/10.1016/j.jmb.2010.08.030
- ↑ Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, Giedroc DP. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010, Sep 8. PMID:20804771 doi:http://dx.doi.org/10.1016/j.jmb.2010.08.030
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532