This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox 123456
From Proteopedia
(heme cofactor green link) |
|||
| Line 1: | Line 1: | ||
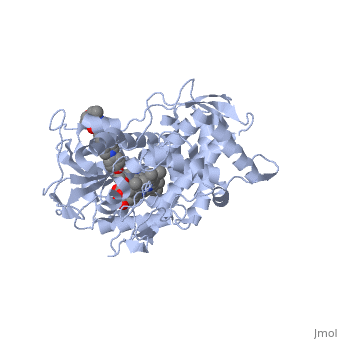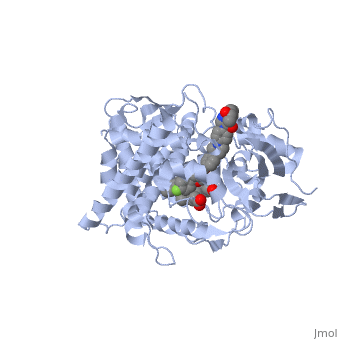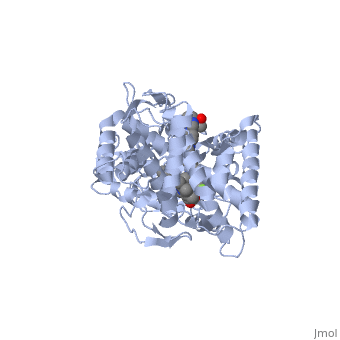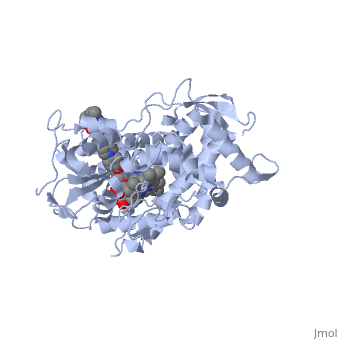
<Structure load='5FSA' size='350' frame='true' align='right' caption='Crystal structure of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans in complex with the antifungal drug posaconazole' /> | <Structure load='5FSA' size='350' frame='true' align='right' caption='Crystal structure of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans in complex with the antifungal drug posaconazole' /> | ||
| - | <scene name='75/756730/Hemegroup/1'>TextToBeDisplayed</scene> | ||
==Introduction== | ==Introduction== | ||
| Line 11: | Line 10: | ||
*IUPAC name: 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one | *IUPAC name: 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one | ||
| - | The primary component of Noxafil, posaconazole (green link) is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines (green link) and that the triazolone sidechain is hydroxylated in the posaconazole structure<ref>PMID: 21059682 </ref>. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the heme cofactor in the active site of CYP450-dependent enzyme lanosterol alpha-demthylase (CYP51) (Groll)(academic.oup.com). The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi (formularyjournal.com). | + | The primary component of Noxafil, posaconazole (green link) is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines (green link) and that the triazolone sidechain is hydroxylated in the posaconazole structure<ref>PMID: 21059682 </ref>. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the <scene name='75/756730/Hemegroup/1'>heme cofactor</scene> in the active site of CYP450-dependent enzyme lanosterol alpha-demthylase (CYP51) (Groll)(academic.oup.com). The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi (formularyjournal.com). |
(explanation of green links/figures?) | (explanation of green links/figures?) | ||
Revision as of 16:39, 19 April 2017
|
Contents |
Introduction
Noxafil, also known as posaconazole, was developed by Schering-Plough in the mid-2000s (acs.org), and is a broad spectrum antifungal drug mainly used to treat fungal infections caused by Candida, Aspergillus, and "Fusarium" species. Noxafil is also often used when other antifungal medicines are not able to be tolerated or if the patient is immunocompromised. Noxafil falls under the triazole class of antifungal drugs and thus works through inhibiting the biosynthesis of ergosterol in the fungal cell membrane, an essential factor that if inhibited, will lead to prevention of cell growth and ultimately death.
Function/Structure
- Chemical Formula: C37H42F2N8O4
- Molecular Weight: 700.792 g/mol
- IUPAC name: 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
The primary component of Noxafil, posaconazole (green link) is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines (green link) and that the triazolone sidechain is hydroxylated in the posaconazole structure[1]. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the in the active site of CYP450-dependent enzyme lanosterol alpha-demthylase (CYP51) (Groll)(academic.oup.com). The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi (formularyjournal.com).
(explanation of green links/figures?)
Mechanism
When administered, Posaconazole acts as a potent and broad-spectrum antifungal drug by binding to the heme cofactor (make green link) located in the active site of CYP450-dependent enzyme lanosterol alpha-demethylase (CYP51) which prevents biosynthesis of ergosterol and causes accumulation of toxic methylated sterol precursor, 14-alpha-methylsterol. Ergosterol is an essential performs in fungal cells how cholesterol does in animal cells, making the cell membrane less permeable. Without it, the cells can no longer proliferate and eventually die because the cell membranes become “leaky”, releasing essential organic components from the cell’s interior and preventing it from performing normal cellular functions (citation). In this way, posaconazole acts as a fungistatic against "Candida" species, and a fungicidal against "Asperigillus" species (formularyjournal.com)
Relevance
Invasive fungal infections, like Candida, Aspergillus, coccidiomycosis, affect patients that are immunocompromised more intensely and are relatively more common in immunocompromised patients. Candida is the most common yeast pathogen while Aspergillus is the most common mold pathogen leading to invasive fungal infections (NCBI). Noxafil oral suspension is the best form of treatment for invasive Candida and Aspergillus infections in patients 13 years and older who are severely immunocompromised. Noxafil is also more effective at preventing invasive fungal infections in immunocompromised patients when compared to other antifungal treatments (fluconazole and itraconazole). Overall, Noxafil displays fewer cases of invasive fungal infections and is also a more affordable treatment for immunocompromised patients.
References
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS. DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011 Jan;39(Database issue):D1035-41. doi:, 10.1093/nar/gkq1126. Epub 2010 Nov 8. PMID:21059682 doi:http://dx.doi.org/10.1093/nar/gkq1126
1. Dekkers, B. G. J., Bakker, M., van der Elst, K. C. M., Sturkenboom, M. G. G., Veringa, A., Span, L. F. R., & Alffenaar, J.-W. C. (2016). Therapeutic Drug Monitoring of Posaconazole: an Update. Current Fungal Infection Reports, 10, 51–61. http://doi.org/10.1007/s12281-016-0255-4 2. Drug Info/Drug Targets: DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS. Nucleic Acids Res. 2011 Jan; 39 (Database issue):D1035-41. | PMID: 21059682 3. Groll, A. H., & Walsh, T. J. (2005). Posaconazole: clinical pharmacology and potential for management of fungal infections. Expert Review of Anti-infective Therapy, 3(4), 467-487. doi:10.1586/14787210.3.4.467 4. Nagappan V, Deresinski S: Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007 Dec 15;45(12):1610-7. doi: 10.1086/523576. [PubMed:18190324 ] 5. National Center for Biotechnology Information. PubChem Compound Database; CID=468595, https://pubchem.ncbi.nlm.nih.gov/compound/468595