Introduction
Adhesin Competence Regulator () is a transcriptional regulator that controls the activation of over seventy genes within the bacteria Streptococcus pneumoniae and is a member of the multiple antibiotic resistance regulator (MarR) protein family [1]. Members of the MarR protein family conserve a number of features including a general triangular shape, a two fold pseudosymmetric homodimer, and a winged helix-turn-helix pattern (wHTH) which can be seen in Figure 1. Consistent with AdcR's identity as a member of the MarR protein family, AdcR exhibits these conserved features. Additionally this structure calls for multiple zinc binding sites that facilitate protein conformational change allowing for DNA binding and regulation through the wHTH domain.
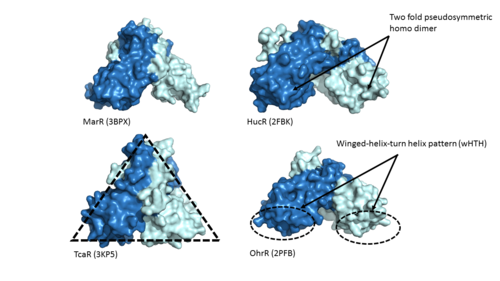
Figure 1. Proteins MarR
(3BPX), HucR
(2FBK), TcaR
(3KP5), and OhrR
(2PFB) are pictured above with conserved features of the MarR protein family highlighted
Contrasting with other members of the MarR family, AdcR is metal dependent. Zinc plays a vital role in organism homeostasis, acting as a co-factor and a regulator of enzymatic activity. However zinc can lead to cell toxicity and deficiency of other vital metals that are also necessary for protein function [2][3]. The importance of AdcR in Streptococcus pneumoniae can be understood provided its ability to regulate zinc transfer proteins within the bacteria. Binding of Zinc allows AdcR to bind DNA and activate the transcription of high-affinity Zinc specific uptake transporters. The binding of Zinc induces a conformational change that allows for a hydrogen bond network between helices of the binding domain. It is believed that this hydrogen bond network is the allosteric activator needed to expose residues that bind the bases along the major groove of the DNA [4].
Structural Overview
One of the two functional domains of AdcR is . This domain is made up of the , the C-terminus of the , and the . This domain is connected to the wHTH domain by the long α5 helix.
DNA Binding
Helix-Turn-Helix Motif
The AdcR MarR transcriptional regulator's structure resembles the other proteins in the same family as mentioned before; however, the most notable differences are found in the winged helix-turn-helix (wHTH) motif that assists in binding DNA. This motif is characteristic of the MarR family and found within the DNA binding domain of the protein. The major groove of DNA is bound to the recognition helix while the wings of the motif grip onto the minor grooves of DNA [4]. Although AdcR is a highly alpha helical protein, the "wings" of the wHTH motif consist of two anti parallel beta strands that are made up of positively charged residues.
The motif is made up of the alpha 2 and alpha 4 helices along with on each side. Only one monomer is shown for clarity purposes. There is one wHTH motif per monomer. The recognition helix, or the alpha 4 helix, binds the major groove of DNA through hydrogen bonding and Van der Waals interactions between exposed bases. The wings of the helix bind the minor groove of DNA while the other helices stabilize the DNA and Protein upon binding. The two anti parallel beta sheets contain several that stabilize this interaction between DNA. The charge map down below highlights the dark blue tips consisting of lysine and arginine residues, which stabilize the negatively charged backbone of DNA.
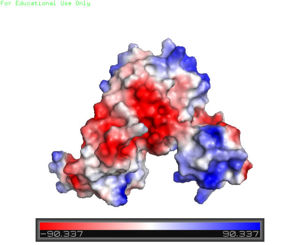
Figure 2. A charge map of AdcR shows the general triangular shape and the positive charged (blue) area on HTH domains
Hydrogen Bond Network
The binding of Zinc allows for the conformational change that induces the binding of DNA in order to activate genes. The binding of Zinc metals creates a hydrogen bond network within the protein that connects the metal binding sites and the DNA binding domain. More importantly, the hydrogen bonding network connects the metal binding pockets to the alpha 4 helix. Alpha 4 helix on each monomer plays a crucial role in binding DNA because it acts as the recognition helix. in the recognition helix recognize a sequence of DNA that is unknown at the moment; however, scientists do know that the hydrogen bond network acts as an allosteric activator for the protein to bind DNA. The hydrogen bond network connects the alpha 2 and alpha 4 helix via hydrogen bonding between specific residues. After zinc is bound, a glutamate (E24) residue from a random coil accepts a hydrogen bond from the carboxamide end of an asparagine (N38) residue from the alpha 2 helix. Then, a glutamine (Q40) residue from alpha 2 helix accepts a hydrogen bond from a serine (S74) residue from the alpha 4 helix [4]. The () is represented by each atom type in the 3D model. The hydrogen bond network is characteristic of the MarR family as a whole.
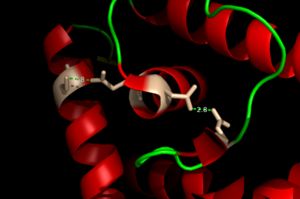
Figure 3. The Hydrogen Bonding Network is shown with dotted green lines approximately 2.8 angstroms between residues. The network consists of 4 major residues as follows from right to left: E24, N38, Q40, S74.
Zn(II) Binding
Zinc-Dependent Transcriptional Regulator AdcR has on each of its two protomers and can bind a total of four Zn(II). The combined with the and make up the . Each protomer has one high affinity site (Binding site 1; KZn1 = 10^12 M; pH 8) and one low affinity binding site (Binding Site 2; KZn2 = 10^7 M; pH 8) [5]. The metal binding pockets of the AdcR MarR transcriptional regulator are made up of the DNA binding domain with the extended α1-α2 loop. The two different Zn(II) binding sites are connected via of H108 and E41.
Binding Site 1
consists of a distorted tetrahedral geometry around Zn(II). The four amino acids involved in zinc binding are E24, H42, H108, and H112. Binding site 1 is the only binding site that plays a significant role in the protein's regulatory function. The ability of binding site 1 to coordinate to the Zn(II) ion is pH dependent. At pH 6 the binding affinity for the Zn(II) ion is 10^9 - 10^10 M^-1, but at pH 8 the binding affinity increases to 10^12 M^-1 [5]. This is due to the charges on the histidines of the binding site. At pH 6, the histidines are positively charged and are not able to interact with the positively charged Zn(II) ion. However, at pH 8 the histidines are neutrally charged and are able to coordinate with Zn(II), which increases the overall binding affinity. The AdcR MarR transcriptional regulator is able to bind Co(II) in binding site 1 in a way that induces similar conformational changes to Zn(II) binding. Co(II) coordination in binding site 1 is able to allosterically activate DNA binding similarly to Zn(II) binding [4].



