We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Noxafil
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
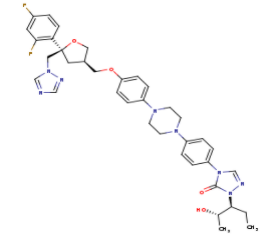
The primary component of Noxafil, <scene name='75/756730/Posaconazole/1'>posaconazole</scene> is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines and that the triazolone sidechain is hydroxylated in the posaconazole structure<ref name="drugbank">Posaconazole. (n.d.). Retrieved from https://www.drugbank.ca/drugs/DB01263 | The primary component of Noxafil, <scene name='75/756730/Posaconazole/1'>posaconazole</scene> is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines and that the triazolone sidechain is hydroxylated in the posaconazole structure<ref name="drugbank">Posaconazole. (n.d.). Retrieved from https://www.drugbank.ca/drugs/DB01263 | ||
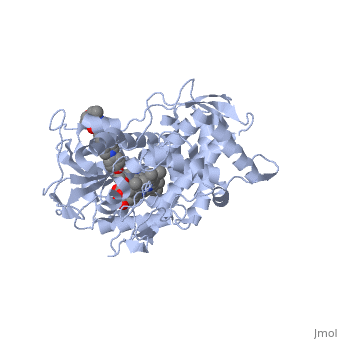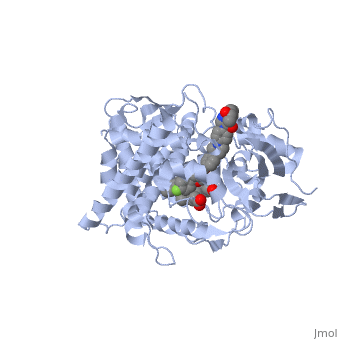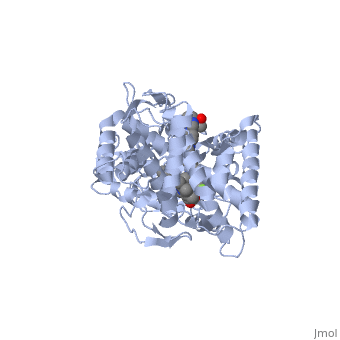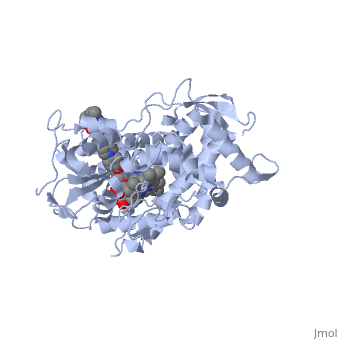
Accession Number: DB01263 </ref>. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the <scene name='75/756730/Hemegroup2/1'>heme cofactor</scene> in the active site of the CYP450-dependent enzyme 14-alpha-demthylase (<scene name='75/756730/Cyp51/1'>CYP51</scene>) <ref name="groll">doi:10.1586/14787210.3.4.467</ref><ref>doi: 10.1086/523576</ref>. The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi <ref name="formularyjournal">Sircar-Ramsewak,, F., Nicolau, D. P., & Kuti, J. L. (2005). Focus on posaconazole: A novel triazole antifungal for the treatment of invasive fungal infections. Formulary Journal - Modern Medicine Network </ref>. The entirety of the scene shows the crystal <scene name='75/756730/Structure/1'>structure</scene> of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans in complex with the antifungal drug posaconazole (PDB ID: 5FSA). | Accession Number: DB01263 </ref>. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the <scene name='75/756730/Hemegroup2/1'>heme cofactor</scene> in the active site of the CYP450-dependent enzyme 14-alpha-demthylase (<scene name='75/756730/Cyp51/1'>CYP51</scene>) <ref name="groll">doi:10.1586/14787210.3.4.467</ref><ref>doi: 10.1086/523576</ref>. The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi <ref name="formularyjournal">Sircar-Ramsewak,, F., Nicolau, D. P., & Kuti, J. L. (2005). Focus on posaconazole: A novel triazole antifungal for the treatment of invasive fungal infections. Formulary Journal - Modern Medicine Network </ref>. The entirety of the scene shows the crystal <scene name='75/756730/Structure/1'>structure</scene> of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans in complex with the antifungal drug posaconazole (PDB ID: 5FSA). | ||
| + | |||
==Mechanism== | ==Mechanism== | ||
When administered, posaconazole acts as a potent and broad-spectrum antifungal drug by binding to the <scene name='75/756730/Hemegroup2/1'>heme cofactor</scene> through an ionic bond between a neutral nitrogen atom on <scene name='75/756730/Posaconazole/1'>posaconazole</scene> and an iron atom on heme located in the active site of <scene name='75/756730/Cyp51/1'>CYP51</scene> . This prevents biosynthesis of ergosterol and causes accumulation of toxic methylated sterol precursor, 14-alpha-methylsterol <ref name="groll"/>. Ergosterol is an essential performs in fungal cells how cholesterol does in animal cells, making the cell membrane less permeable. Without it, the cells can no longer proliferate and eventually die because the cell membranes become “leaky”, releasing essential organic components from the cell’s interior and preventing it from performing normal cellular functions (citation?). In this way, posaconazole acts as a fungistatic against ''Candida'' species, and a fungicidal against ''Aspergillus'' species <ref name="formularyjournal" />. | When administered, posaconazole acts as a potent and broad-spectrum antifungal drug by binding to the <scene name='75/756730/Hemegroup2/1'>heme cofactor</scene> through an ionic bond between a neutral nitrogen atom on <scene name='75/756730/Posaconazole/1'>posaconazole</scene> and an iron atom on heme located in the active site of <scene name='75/756730/Cyp51/1'>CYP51</scene> . This prevents biosynthesis of ergosterol and causes accumulation of toxic methylated sterol precursor, 14-alpha-methylsterol <ref name="groll"/>. Ergosterol is an essential performs in fungal cells how cholesterol does in animal cells, making the cell membrane less permeable. Without it, the cells can no longer proliferate and eventually die because the cell membranes become “leaky”, releasing essential organic components from the cell’s interior and preventing it from performing normal cellular functions (citation?). In this way, posaconazole acts as a fungistatic against ''Candida'' species, and a fungicidal against ''Aspergillus'' species <ref name="formularyjournal" />. | ||
| + | |||
== Pharmaceutical Information == | == Pharmaceutical Information == | ||
| Line 22: | Line 24: | ||
Posaconazole has not been found to be have significant dose-limiting toxicity and has more reduced drug-drug interactions than many other antifungals (formularyjournal) | Posaconazole has not been found to be have significant dose-limiting toxicity and has more reduced drug-drug interactions than many other antifungals (formularyjournal) | ||
It should be taken with food to help absorption and is generally taken 3 times a day in 200 mg doses. For fungi that have been unmanageable through other treatments, 800 mg are given daily 2 or 4 times a day to treat infection <ref name="greer"> Greer ND. Posaconazole (Noxafil): a new triazole antifungal agent. Proceedings (Baylor University Medical Center). 2007;20(2):188-196. PMID: PMC1849883 </ref> | It should be taken with food to help absorption and is generally taken 3 times a day in 200 mg doses. For fungi that have been unmanageable through other treatments, 800 mg are given daily 2 or 4 times a day to treat infection <ref name="greer"> Greer ND. Posaconazole (Noxafil): a new triazole antifungal agent. Proceedings (Baylor University Medical Center). 2007;20(2):188-196. PMID: PMC1849883 </ref> | ||
| + | |||
== Relevance == | == Relevance == | ||
Revision as of 22:01, 19 April 2017
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Madelyn Smith, Gianna Cutrone, Michal Harel, Kelley Barker, Hannah Ackleson