We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox1996
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Structure == | == Structure == | ||
| - | + | ||
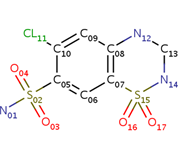
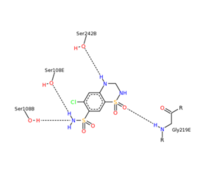
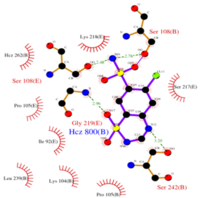
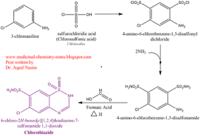
<scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash po int of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2 & 3). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 4)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash po int of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2 & 3). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 4)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# | ||
</ref>. | </ref>. | ||
Revision as of 22:06, 19 April 2017
Diuril (Chlorothiazide)
| |||||||||||
References
- ↑ 1.0 1.1 EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ
- ↑ Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html
- ↑ EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6
- ↑ Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html#
- ↑ The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ . Unlike other hypotensive drugs that lower blood pressure equally in both hypertensive and normotensive patients, Diuril reduces the blood pressure of patients in the hypertensive state only. It, therefore, possesses considerable specificity. Diuril was also favored over other hypotensive drugs due to ease in its administration; it was delivered orally and required no hospitalization or testing to monitor for immediate adverse effects. When combined with agents such as ganglionic blockers, reserpine, and hydralazine, the drug's efficacy was synergistically increased while its respective toxicities were minimized <ref> Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794. </li> <li id="cite_note-nine-7">↑ <sup>[[#cite_ref-nine_7-0|8.0]]</sup> <sup>[[#cite_ref-nine_7-1|8.1]]</sup> Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html </li> <li id="cite_note-ten-8">[[#cite_ref-ten_8-0|↑]] RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103 </li> <li id="cite_note-eleven-9">[[#cite_ref-eleven_9-0|↑]] Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743. </li> <li id="cite_note-twelve-10">↑ <sup>[[#cite_ref-twelve_10-0|11.0]]</sup> <sup>[[#cite_ref-twelve_10-1|11.1]]</sup> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </li> <li id="cite_note-thirteen-11">[[#cite_ref-thirteen_11-0|↑]] AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html </li> <li id="cite_note-fourteen-12">[[#cite_ref-fourteen_12-0|↑]] The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224 </li> <li id="cite_note-fifteen-13">[[#cite_ref-fifteen_13-0|↑]] Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265. </li></ol></ref>