We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox1996
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Structure == | == Structure == | ||
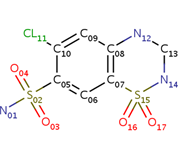
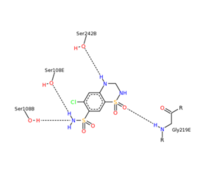
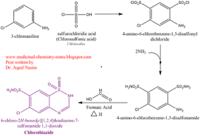
| - | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) | + | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1). It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of a benzothiadiazine, sulfonamide, chloride, dihydro, and dioxide group. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 3)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# |
</ref>. | </ref>. | ||
| Line 12: | Line 12: | ||
== Function and Mechanism== | == Function and Mechanism== | ||
| - | Chlorothiazide has diuretic and anti-hypertensive properties. Chlorothiazide acts as a diuretic, which means that it inhibits the reabsorption of chloride. This occurs at the distal tubules via the sodium-chloride co-transporter. The result of this is an increased excretion of sodium, chlorine, and water. Chlorothiazide also inhibits sodium ion transport across the renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter. More specifically, chlorothiazide targets<scene name='75/756546/Solute_carrier_family_12_membe/2'> solute carrier family 12 member 3 </scene>. The result of this is an increase in the excretion of potassium using the sodium-potassium exchange system. As for the anti-hypertensive properties of chlorothiazide, the mechanism is not quite as known. It is thought that vasodilation is caused by the activation of calcium-activated potassium channels (KCa) and the inhibition of carbonic anhydrases | + | Chlorothiazide has diuretic and anti-hypertensive properties. Chlorothiazide acts as a diuretic, which means that it inhibits the reabsorption of chloride. This occurs at the distal tubules via the sodium-chloride co-transporter. The result of this is an increased excretion of sodium, chlorine, and water. Chlorothiazide also inhibits sodium ion transport across the renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter. More specifically, chlorothiazide targets<scene name='75/756546/Solute_carrier_family_12_membe/2'> solute carrier family 12 member 3</scene>. The result of this is an increase in the excretion of potassium using the sodium-potassium exchange system. As for the anti-hypertensive properties of chlorothiazide, the mechanism is not quite as known. It is thought that vasodilation is caused by the activation of calcium-activated potassium channels (KCa) and the inhibition of carbonic anhydrases<ref name = "five"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 |
</ref>. | </ref>. | ||
Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_1/1'>carbonic anhydrase 1</scene> to perform the reversible hydration of carbon dioxide.<scene name='75/756546/Carbonic_anhydrase_2/1'> Carbonic anhydrase 2</scene> is also targeted to perform the reversible hydration of carbon dioxide, regulate the fluid secretion of the anterior chamber of the eye and the intracellular pH in the duodenal upper villous epithelium during proton-coupled peptide absorption, and stimulate the chloride-bicarbonate exchange activity of SLC26A6. Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_4/1'>carbonic anhydrase 4</scene> to perform the reversible hydration of carbon dioxide, stimulate the sodium/bicarbonate transporter activity of SLC4A4, and remove acid overload from the retina and retina epithelium <ref name = "six"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 | Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_1/1'>carbonic anhydrase 1</scene> to perform the reversible hydration of carbon dioxide.<scene name='75/756546/Carbonic_anhydrase_2/1'> Carbonic anhydrase 2</scene> is also targeted to perform the reversible hydration of carbon dioxide, regulate the fluid secretion of the anterior chamber of the eye and the intracellular pH in the duodenal upper villous epithelium during proton-coupled peptide absorption, and stimulate the chloride-bicarbonate exchange activity of SLC26A6. Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_4/1'>carbonic anhydrase 4</scene> to perform the reversible hydration of carbon dioxide, stimulate the sodium/bicarbonate transporter activity of SLC4A4, and remove acid overload from the retina and retina epithelium <ref name = "six"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 | ||
| Line 22: | Line 22: | ||
===Hypertension=== | ===Hypertension=== | ||
| - | Although Diuril was introduced as an anti-diuretic drug, its antihypertensive properties were discovered when two researchers from Boston and Washington D.C administered the drug to patients suffering from congestive heart failure, edema and severe hypertension, and noted a dramatic reduction in high blood pressure <ref name = "seven" Freis, E., Wanko, A., Wilson, I., and Parrish, A.E. (1985) Treatment of essential diabetes with Chlorothiazide (Diuril), J. Am. Med. Assoc 166, 137-140 </ref>. Unlike other hypotensive drugs that lower blood pressure equally in both hypertensive and normotensive patients, Diuril reduces the blood pressure of patients in the hypertensive state only. It, therefore, possesses considerable specificity. Diuril was also favored over other hypotensive drugs due to ease in its administration; it was delivered orally and required no hospitalization or testing to monitor for immediate adverse effects. When combined with agents such as ganglionic blockers, reserpine, and hydralazine, the drug's efficacy was synergistically increased while its respective toxicities were minimized <ref name = "eight"> Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794. </ref>. | + | Although Diuril was introduced as an anti-diuretic drug, its antihypertensive properties were discovered when two researchers from Boston and Washington D.C administered the drug to patients suffering from congestive heart failure, edema and severe hypertension, and noted a dramatic reduction in high blood pressure <ref name = "seven" > Freis, E., Wanko, A., Wilson, I., and Parrish, A.E. (1985) Treatment of essential diabetes with Chlorothiazide (Diuril), J. Am. Med. Assoc 166, 137-140 </ref>. Unlike other hypotensive drugs that lower blood pressure equally in both hypertensive and normotensive patients, Diuril reduces the blood pressure of patients in the hypertensive state only. It, therefore, possesses considerable specificity. Diuril was also favored over other hypotensive drugs due to ease in its administration; it was delivered orally and required no hospitalization or testing to monitor for immediate adverse effects. When combined with agents such as ganglionic blockers, reserpine, and hydralazine, the drug's efficacy was synergistically increased while its respective toxicities were minimized <ref name = "eight"> Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794. </ref>. |
===Decrease Edema=== | ===Decrease Edema=== | ||
Revision as of 22:20, 19 April 2017
Diuril (Chlorothiazide)
| |||||||||||
References
- ↑ EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ
- ↑ Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html
- ↑ EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6
- ↑ Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html#
- ↑ The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ Freis, E., Wanko, A., Wilson, I., and Parrish, A.E. (1985) Treatment of essential diabetes with Chlorothiazide (Diuril), J. Am. Med. Assoc 166, 137-140
- ↑ Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794.
- ↑ 9.0 9.1 Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html
- ↑ RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103
- ↑ Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743.
- ↑ 12.0 12.1 Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones
- ↑ AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html
- ↑ The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224
- ↑ Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265.