We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox1996
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Structure == | == Structure == | ||
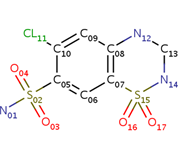
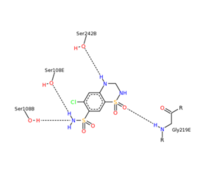
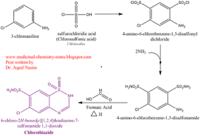
| - | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of a benzothiadiazine, sulfonamide, chloride, dihydro, and dioxide group. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 3)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# | + | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of a benzothiadiazine, sulfonamide, chloride, dihydro, and dioxide group. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2) <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 3)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# |
</ref>. | </ref>. | ||
| Line 14: | Line 14: | ||
Chlorothiazide has diuretic and anti-hypertensive properties. Chlorothiazide acts as a diuretic, which means that it inhibits the reabsorption of chloride. This occurs at the distal tubules via the sodium-chloride co-transporter. The result of this is an increased excretion of sodium, chlorine, and water. Chlorothiazide also inhibits sodium ion transport across the renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter. More specifically, chlorothiazide targets<scene name='75/756546/Solute_carrier_family_12_membe/2'> solute carrier family 12 member 3</scene>. The result of this is an increase in the excretion of potassium using the sodium-potassium exchange system. As for the anti-hypertensive properties of chlorothiazide, the mechanism is not quite as known. It is thought that vasodilation is caused by the activation of calcium-activated potassium channels (KCa) and the inhibition of carbonic anhydrases<ref name = "five"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 | Chlorothiazide has diuretic and anti-hypertensive properties. Chlorothiazide acts as a diuretic, which means that it inhibits the reabsorption of chloride. This occurs at the distal tubules via the sodium-chloride co-transporter. The result of this is an increased excretion of sodium, chlorine, and water. Chlorothiazide also inhibits sodium ion transport across the renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter. More specifically, chlorothiazide targets<scene name='75/756546/Solute_carrier_family_12_membe/2'> solute carrier family 12 member 3</scene>. The result of this is an increase in the excretion of potassium using the sodium-potassium exchange system. As for the anti-hypertensive properties of chlorothiazide, the mechanism is not quite as known. It is thought that vasodilation is caused by the activation of calcium-activated potassium channels (KCa) and the inhibition of carbonic anhydrases<ref name = "five"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 | ||
</ref>. | </ref>. | ||
| - | Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_1/1'>carbonic anhydrase 1</scene> to perform the reversible hydration of carbon dioxide.<scene name='75/756546/Carbonic_anhydrase_2/1'> Carbonic anhydrase 2</scene> is also targeted to perform the reversible hydration of carbon dioxide, regulate the fluid secretion of the anterior chamber of the eye and the intracellular pH in the duodenal upper villous epithelium during proton-coupled peptide absorption, and stimulate the chloride-bicarbonate exchange activity of SLC26A6. Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_4/1'>carbonic anhydrase 4</scene> to perform the reversible hydration of carbon dioxide, stimulate the sodium/bicarbonate transporter activity of SLC4A4, and remove acid overload from the retina and retina epithelium <ref name = " | + | Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_1/1'>carbonic anhydrase 1</scene> to perform the reversible hydration of carbon dioxide.<scene name='75/756546/Carbonic_anhydrase_2/1'> Carbonic anhydrase 2</scene> is also targeted to perform the reversible hydration of carbon dioxide, regulate the fluid secretion of the anterior chamber of the eye and the intracellular pH in the duodenal upper villous epithelium during proton-coupled peptide absorption, and stimulate the chloride-bicarbonate exchange activity of SLC26A6. Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_4/1'>carbonic anhydrase 4</scene> to perform the reversible hydration of carbon dioxide, stimulate the sodium/bicarbonate transporter activity of SLC4A4, and remove acid overload from the retina and retina epithelium <ref name = "five"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 |
</ref>. | </ref>. | ||
| Line 22: | Line 22: | ||
===Hypertension=== | ===Hypertension=== | ||
| - | Although Diuril was introduced as an anti-diuretic drug, its anti-hypertensive properties were discovered when two researchers from Boston and Washington D.C administered the drug to patients suffering from congestive heart failure, edema, and severe hypertension, and noted a dramatic reduction in high blood pressure <ref name = " | + | Although Diuril was introduced as an anti-diuretic drug, its anti-hypertensive properties were discovered when two researchers from Boston and Washington D.C administered the drug to patients suffering from congestive heart failure, edema, and severe hypertension, and noted a dramatic reduction in high blood pressure <ref name = "six" > Freis, E., Wanko, A., Wilson, I., and Parrish, A.E. (1985) Treatment of essential diabetes with Chlorothiazide (Diuril), J. Am. Med. Assoc 166, 137-140 </ref>. Unlike other hypotensive drugs that lower blood pressure equally in both hypertensive and normotensive patients, Diuril reduces the blood pressure of patients in the hypertensive state only. It therefore possesses considerable specificity. Diuril was also favored over other hypotensive drugs because of its ease of administration; it could be delivered orally, and required no hospitalization or testing to monitor for immediate adverse effects. When combined with agents such as ganglionic blockers, reserpine, and hydralazine, the drug's efficacy was synergistically increased while its respective toxicities were minimized <ref name = "seven"> Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794. </ref>. |
===Decrease Edema=== | ===Decrease Edema=== | ||
| - | Diuril functions as a thiazoide diuretic to treat edema in patients diagnosed with cirrhosis of the liver, congestive heart failure, or with disorders associated with the kidney<ref name = " | + | Diuril functions as a thiazoide diuretic to treat edema in patients diagnosed with cirrhosis of the liver, congestive heart failure, or with disorders associated with the kidney<ref name = "eight"> Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html </ref>. As a diuretic, Diuril is responsible for inducing fluid loss <ref name = "nine"> RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103 </ref>. Its role in adjunctive therapy is to assist the primary form of treatment in handling symptoms associated with a disease. In some cases, a short-term treatment of Diuril may be used to treat edema occurring during pregnancy associated with hypervolemia that is causing discomfort in the patient<ref name = "eight"> Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html |
</ref>. | </ref>. | ||
===Diabetes Insipidus=== | ===Diabetes Insipidus=== | ||
| - | Diabetes Insipidus is a rare disorder caused by the kidney’s inability to retain water. There are four different types of diabetes Insipidus; Central, Nephrogenic, Dipsogenic and Gestational. High concentrations of sodium and potassium are characteristic of all types of Diabetes Insipidus. Treatment with Diuril demonstrates a decrease in saluresis, i.e the excretion of sodium and chloride, within approximately eight hours following its initial dose, and a decrease in kaliuresis, the excretion of potassium ions, approximately fifteen hours following its initial dose.<ref name = " | + | Diabetes Insipidus is a rare disorder caused by the kidney’s inability to retain water. There are four different types of diabetes Insipidus; Central, Nephrogenic, Dipsogenic and Gestational. High concentrations of sodium and potassium are characteristic of all types of Diabetes Insipidus. Treatment with Diuril demonstrates a decrease in saluresis, i.e the excretion of sodium and chloride, within approximately eight hours following its initial dose, and a decrease in kaliuresis, the excretion of potassium ions, approximately fifteen hours following its initial dose.<ref name = "ten"> Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743. </ref>. |
===Kidney Stones=== | ===Kidney Stones=== | ||
| - | Kidneys are needed to filter fluids and waste from the body to produce urine. Sometimes there are high levels of chemicals in the urine that form crystals and the crystals eventually become large enough to form stones in the kidney <ref name = " | + | Kidneys are needed to filter fluids and waste from the body to produce urine. Sometimes there are high levels of chemicals in the urine that form crystals and the crystals eventually become large enough to form stones in the kidney <ref name = "eleven"> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </ref>. Chlorothiazide can help prevent against calcium kidney stones with patients that have high calcium concentrations in their blood.<ref name = "twelve"> AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html </ref> Thiazides (Diuril is an example of one) can cause potassium loss as well, which reduces citrate levels. Lowered citrate levels can increase the risk of kidney stones, so Diuril needs to be taken in conjunction with potassium-citrate pills <ref name = "eleven"> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </ref>. |
===Side Effects=== | ===Side Effects=== | ||
| - | One main side effect that should be noted when taking Diuril is the introduction of purpura, or excessive bruising and superficial bleeding, typically on the legs <ref name = " | + | One main side effect that should be noted when taking Diuril is the introduction of purpura, or excessive bruising and superficial bleeding, typically on the legs <ref name = "thirteen"> The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224 </ref>. There are many different types of purpura, but the general bleeding of small vessels and inflammation hold true. Some patients, when taking Diuril more regularly, as in twice a day for a specific number of weeks, will exhibit purpura. This can be treated typically with bedrest, minor medications, and discontinued use of chlorothiazide. One study from the Mayo Clinic showed that, after discontinuing use of chlorothiazide and then readministering a single dose, purpura reappeared quite rapidly, leading to inferences that chlorothiazide use was what originally brought on the purpura <ref name = "fourteen"> Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265. </ref>. |
Revision as of 22:34, 19 April 2017
Diuril (Chlorothiazide)
| |||||||||||
References
- ↑ 1.0 1.1 EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ
- ↑ Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html
- ↑ 3.0 3.1 EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6
- ↑ Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html#
- ↑ 5.0 5.1 The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ Freis, E., Wanko, A., Wilson, I., and Parrish, A.E. (1985) Treatment of essential diabetes with Chlorothiazide (Diuril), J. Am. Med. Assoc 166, 137-140
- ↑ Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794.
- ↑ 8.0 8.1 Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html
- ↑ RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103
- ↑ Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743.
- ↑ 11.0 11.1 Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones
- ↑ AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html
- ↑ The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224
- ↑ Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265.