Sandbox Reserved 1069
From Proteopedia
| Line 11: | Line 11: | ||
===Electrostatic Interactions=== | ===Electrostatic Interactions=== | ||
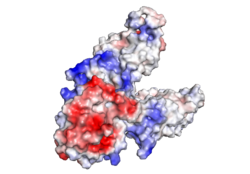
| - | [[Image:ElectrostaticV2.png|250px|right|thumb|Electrostatic Charge Distribution]]<scene name='69/694236/Electrostaticv2/1'>Charge Distribution</scene> along the exterior surface of the protein, shown in Figure 1, is primarily neutral (white) for the TMDs, but transitions to positive (blue) near the location of the charge interlock and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation within the cell membrane. Binding sites A, B, and C, as well as the CTDs of both monomers, all possess a high negative charge (red) relative to the other charges present, facilitating the binding and releasing of Zn<sup>2+</sup> ions. The two CTDs are held together by the charge interlock and hydrophobic interactions of the TMDs, despite their electrostatic repulsion. Upon the release of Zn<sup>2+</sup> ions, the CTDs undergo alterations to electronegativity, which enables domain separation. | + | [[Image:ElectrostaticV2.png|250px|right|thumb|Figure 1. Electrostatic Charge Distribution]]<scene name='69/694236/Electrostaticv2/1'>Charge Distribution</scene> along the exterior surface of the protein, shown in Figure 1, is primarily neutral (white) for the TMDs, but transitions to positive (blue) near the location of the charge interlock and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation within the cell membrane. Binding sites A, B, and C, as well as the CTDs of both monomers, all possess a high negative charge (red) relative to the other charges present, facilitating the binding and releasing of Zn<sup>2+</sup> ions. The two CTDs are held together by the charge interlock and hydrophobic interactions of the TMDs, despite their electrostatic repulsion. Upon the release of Zn<sup>2+</sup> ions, the CTDs undergo alterations to electronegativity, which enables domain separation. |
===Interlocking Salt Bridge=== | ===Interlocking Salt Bridge=== | ||
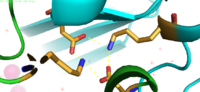
| - | The <scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene> formation between Lys77 and Asp207 of each domain of YiiP forms an interlocking interaction that acts as the pivot point of the conformational change that drives the function of YiiP. Interlocking interactions are disrupted when Zn<sup>2+</sup> is bound, due to movement of the antiparallel helices, causing a conformational shift in YiiP. The salt bridge also aids in holding the two monomers together, where [[Image:Saltbridge.png|200px|left|thumb|Lys77 and Asp207 Salt Bridges]]<scene name='75/756372/Besthydrophobiczoom1/1'>hydrophobic</scene> residues around the salt bridge further stabilize the two domains in the v-shaped void where the domains connect. This prevents degradation of the protein's interlock via interactions with the environment. | + | The <scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene> formation between Lys77 and Asp207 of each domain of YiiP forms an interlocking interaction that acts as the pivot point of the conformational change that drives the function of YiiP. Interlocking interactions are disrupted when Zn<sup>2+</sup> is bound, due to movement of the antiparallel helices, causing a conformational shift in YiiP. The salt bridge also aids in holding the two monomers together, where [[Image:Saltbridge.png|200px|left|thumb|Figure 2. Lys77 and Asp207 Salt Bridges]]<scene name='75/756372/Besthydrophobiczoom1/1'>hydrophobic</scene> residues around the salt bridge further stabilize the two domains in the v-shaped void where the domains connect. This prevents degradation of the protein's interlock via interactions with the environment. |
=== Zn<sup>2+</sup> Binding Sites === | === Zn<sup>2+</sup> Binding Sites === | ||
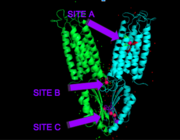
| - | [[Image:activesites.png|thumb|right|]] | + | [[Image:activesites.png|thumb|right|Figure 3.]]Each Yiip monomer contains three Zn<sup>2+</sup> binding sites. There is an active site (Site A), and two cytoplasmic binding sites (Site B and C). It was found that only site A and C are conserved, while the function of Site B is not well defined, though it is believed that it plays a role in subunit dimerization. |
| - | Each Yiip monomer contains three Zn<sup>2+</sup> binding sites. There is an active site (Site A), and two cytoplasmic binding sites (Site B and C). It was found that only site A and C are conserved, while the function of Site B is not well defined, though it is believed that it plays a role in subunit dimerization. | + | |
| Line 28: | Line 27: | ||
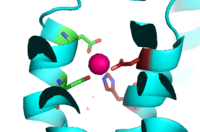
<scene name='69/694236/Binding_site_a_transparent/1'>Binding site A</scene> is in the center of the transmembrane domain, attached and confined via residues from the TM2 and TM5 helices. The TM2 domain has <scene name='69/694236/Asp45/1'>Asp45</scene> and <scene name='69/694235/Asp49/1'>Asp49</scene>, and the TM5 has <scene name='69/694235/His153/1'>His153</scene> and <scene name='69/694235/Asp153/2'>Asp157</scene>, which facilitate the binding and releasing of Zn<sup>2+</sup> within the sites. The TM5 helix is significantly shorter than the other 5 helices around it, and this length forms a cavity in the membrane. Site A is one of Yiip's active sites, where Zn<sup>2+</sup> is able to attach and eventually exit the cell via proton transport. This particular site has an ideal tetrahedron among its residues, which is preferred for Zn<sup>2+</sup> binding. | <scene name='69/694236/Binding_site_a_transparent/1'>Binding site A</scene> is in the center of the transmembrane domain, attached and confined via residues from the TM2 and TM5 helices. The TM2 domain has <scene name='69/694236/Asp45/1'>Asp45</scene> and <scene name='69/694235/Asp49/1'>Asp49</scene>, and the TM5 has <scene name='69/694235/His153/1'>His153</scene> and <scene name='69/694235/Asp153/2'>Asp157</scene>, which facilitate the binding and releasing of Zn<sup>2+</sup> within the sites. The TM5 helix is significantly shorter than the other 5 helices around it, and this length forms a cavity in the membrane. Site A is one of Yiip's active sites, where Zn<sup>2+</sup> is able to attach and eventually exit the cell via proton transport. This particular site has an ideal tetrahedron among its residues, which is preferred for Zn<sup>2+</sup> binding. | ||
| - | [[Image:Binding_site_A.fw.png|200px|left|thumb|Binding Site A showing TM2 domain (left) and TM5 domain (right). The Asp45 and Asp49 as well as the His153 and Asp157 are the coordination residues in the acitve site]] | + | [[Image:Binding_site_A.fw.png|200px|left|thumb|Figure 4. Binding Site A showing TM2 domain (left) and TM5 domain (right). The Asp45 and Asp49 as well as the His153 and Asp157 are the coordination residues in the acitve site]] |
It is important to note that the structure of this binding site is rigid because of the coordination of the Zn<sup>2+</sup> between the four residues. This rigidity is indicative that any slight shift on either of the helices will cause a drastic readjustment of the coordination of Zn<sup>2+</sup>. In addition, there are no outer-shell constraints to hold the residues in place, which means that with a readjustment of the molecule, there is no energy being expended to bind or release another Zn<sup>2+</sup> molecule. Therefore, the Zn<sup>2+</sup> is able to rapidly release and bind a new Zn<sup>2+</sup> with a simple reorientation or shift of the molecule. This rapid on/off bind and release mechanism is the regulator of homeostatic levels of Zn<sup>2+</sup> in the cell, which is significantly faster than other Zn<sup>2+</sup> exchange rate proteins by several orders of magnitude. | It is important to note that the structure of this binding site is rigid because of the coordination of the Zn<sup>2+</sup> between the four residues. This rigidity is indicative that any slight shift on either of the helices will cause a drastic readjustment of the coordination of Zn<sup>2+</sup>. In addition, there are no outer-shell constraints to hold the residues in place, which means that with a readjustment of the molecule, there is no energy being expended to bind or release another Zn<sup>2+</sup> molecule. Therefore, the Zn<sup>2+</sup> is able to rapidly release and bind a new Zn<sup>2+</sup> with a simple reorientation or shift of the molecule. This rapid on/off bind and release mechanism is the regulator of homeostatic levels of Zn<sup>2+</sup> in the cell, which is significantly faster than other Zn<sup>2+</sup> exchange rate proteins by several orders of magnitude. | ||
| Line 38: | Line 37: | ||
==Mechanism of Transport== | ==Mechanism of Transport== | ||
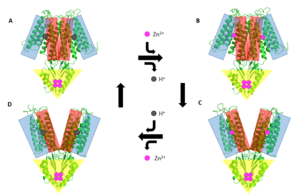
| - | [[Image:YiiP_Mechanism.png|300px|right|thumb| General mechanism that YiiP uses to transport Zn2+ from the cytoplasm to the periplasm. This mechanism involves 2 major conformations; the inward-facing conformation (A & B) and the outward-facing conformation (C & D). Helices TM1, TM2, TM4, and TM5 (blue) are shown pivoting relative to helices TM3 & TM6 (red). The CTD (yellow) does not move during this conformation change as it it held together tightly by binding Zn<sup>2+</sup>.]] | + | [[Image:YiiP_Mechanism.png|300px|right|thumb| Figure 5. General mechanism that YiiP uses to transport Zn2+ from the cytoplasm to the periplasm. This mechanism involves 2 major conformations; the inward-facing conformation (A & B) and the outward-facing conformation (C & D). Helices TM1, TM2, TM4, and TM5 (blue) are shown pivoting relative to helices TM3 & TM6 (red). The CTD (yellow) does not move during this conformation change as it it held together tightly by binding Zn<sup>2+</sup>.]] |
YiiP's ability to export Zn<sup>2+</sup> from the cytoplasm is best described as an alternating access mechanism with Zn<sup>2+</sup>/H<sup>+</sup> antiport. YiiP has 2 major structural conformations as shown by the crystallized structures [http://proteopedia.org/wiki/index.php/3h90 3H90] and [http://proteopedia.org/wiki/index.php/3j1z 3J1Z] <ref>PMID:23341604</ref> (a YiiP homolog derived from ''Shewanella oneidensis''). 3H90 shows YiiP in its outward-facing conformation with Zn<sup>2+</sup> present and 3J1Z which shows the YiiP homolog in an inward-facing conformation where there is no Zn<sup>2+</sup> present. | YiiP's ability to export Zn<sup>2+</sup> from the cytoplasm is best described as an alternating access mechanism with Zn<sup>2+</sup>/H<sup>+</sup> antiport. YiiP has 2 major structural conformations as shown by the crystallized structures [http://proteopedia.org/wiki/index.php/3h90 3H90] and [http://proteopedia.org/wiki/index.php/3j1z 3J1Z] <ref>PMID:23341604</ref> (a YiiP homolog derived from ''Shewanella oneidensis''). 3H90 shows YiiP in its outward-facing conformation with Zn<sup>2+</sup> present and 3J1Z which shows the YiiP homolog in an inward-facing conformation where there is no Zn<sup>2+</sup> present. | ||
When YiiP is saturated with Zn<sup>2+</sup> it favors the <scene name='69/694236/Outward-facinggreen/1'>outward-facing conformation</scene> whereas when active sites are either empty or bound to H<sup>+</sup> the <scene name='69/694236/Inward-facinggreen/1'>inward-facing conformation</scene> is favored. This drives the export of Zn<sup>2+</sup> from the cytoplasm to the periplasm. Although YiiP exists as a homodimer both monomers can undergo conformation change independent of one other to | When YiiP is saturated with Zn<sup>2+</sup> it favors the <scene name='69/694236/Outward-facinggreen/1'>outward-facing conformation</scene> whereas when active sites are either empty or bound to H<sup>+</sup> the <scene name='69/694236/Inward-facinggreen/1'>inward-facing conformation</scene> is favored. This drives the export of Zn<sup>2+</sup> from the cytoplasm to the periplasm. Although YiiP exists as a homodimer both monomers can undergo conformation change independent of one other to | ||
| Line 46: | Line 45: | ||
===Zn<sup>2+</sup> Induced Conformation Change=== | ===Zn<sup>2+</sup> Induced Conformation Change=== | ||
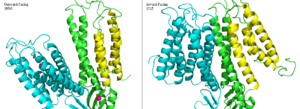
| - | Zinc induced conformation changes in the TMD and CTD leads to the major <scene name='69/694236/Outward-facinggreen/1'>outward-facing</scene> and <scene name='69/694236/Inward-facinggreen/1'>inward-facing conformations</scene>. [[Image:InwardVsOutward.png|300px|right|thumb| Side by side comparison of one monomer for the the outward-facing conformation of 3H90 and the inward-facing conformation of 3J1Z. TM1, TM2, TM4, and TM5 (yellow) pivot around TM3 and TM6 (green). The helices of the other half of the homodimer (blue) function identically. | + | Zinc induced conformation changes in the TMD and CTD leads to the major <scene name='69/694236/Outward-facinggreen/1'>outward-facing</scene> and <scene name='69/694236/Inward-facinggreen/1'>inward-facing conformations</scene>. [[Image:InwardVsOutward.png|300px|right|thumb| Figure 6. Side by side comparison of one monomer for the the outward-facing conformation of 3H90 and the inward-facing conformation of 3J1Z. TM1, TM2, TM4, and TM5 (yellow) pivot around TM3 and TM6 (green). The helices of the other half of the homodimer (blue) function identically. |
]] | ]] | ||
The conformation change directly involved with Zn<sup>2+</sup>/H<sup>+</sup> antiport occurs in the TMD as helix pivoting controls what environment site A is available to. Conformation change occurs when the transmembrane helix pairs TM1, TM2, TM4, and TM5 pivot around cation binding site A.<ref>PMID:23341604</ref> | The conformation change directly involved with Zn<sup>2+</sup>/H<sup>+</sup> antiport occurs in the TMD as helix pivoting controls what environment site A is available to. Conformation change occurs when the transmembrane helix pairs TM1, TM2, TM4, and TM5 pivot around cation binding site A.<ref>PMID:23341604</ref> | ||
It is believed that the energy for TMD conformation change comes from energy of binding each substrate. Changing to the outward from the inward-facing conformation causes a shift in <scene name='69/694233/Transmembrane_helix_5/2'>TM5</scene> which disrupts the tetrahedral geometry of active site A. This in turn decreases binding affinity site A has for Zn<sup>2+</sup> making export to the periplasm possible. After Zn<sup>2+</sup> is exported and site A is either empty or bound to H<sup>+</sup>, the protein's conformation changes to the favored inward-facing conformation. | It is believed that the energy for TMD conformation change comes from energy of binding each substrate. Changing to the outward from the inward-facing conformation causes a shift in <scene name='69/694233/Transmembrane_helix_5/2'>TM5</scene> which disrupts the tetrahedral geometry of active site A. This in turn decreases binding affinity site A has for Zn<sup>2+</sup> making export to the periplasm possible. After Zn<sup>2+</sup> is exported and site A is either empty or bound to H<sup>+</sup>, the protein's conformation changes to the favored inward-facing conformation. | ||
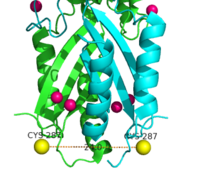
| - | [[Image:FRET.png|200px|left|thumb| Labeled Cysteine resides measured with FRET showed the distance of the CTD of each monomer to be 24.0Å when saturated with Zn<sup>2+</sup>. Decrease in the Cys-Cys distance is indicative that both CTDs of YiiP were brought closer together.]] | + | [[Image:FRET.png|200px|left|thumb| Figure 7. Labeled Cysteine resides measured with FRET showed the distance of the CTD of each monomer to be 24.0Å when saturated with Zn<sup>2+</sup>. Decrease in the Cys-Cys distance is indicative that both CTDs of YiiP were brought closer together.]] |
In contrast the main purpose of conformation change in the CTD is to stabilize the YiiP dimer and to act as a Zn<sup>2+</sup> sensor. | In contrast the main purpose of conformation change in the CTD is to stabilize the YiiP dimer and to act as a Zn<sup>2+</sup> sensor. | ||
This is possible because of the flexible loop that links the TMD and the CTD. This loop harbors the <scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene> which serves as a hinge that allows movement of the CTD. Using [https://en.wikipedia.org/wiki/F%C3%B6rster_resonance_energy_transfer FRET] to measure the distance between the CTD of each monomer fluorescence quenching was observed as the concentration Zn<sup>2+</sup> increased, which supports that idea that Zn<sup>2+</sup> induces a stabilizing conformation change in the CTD.<ref>PMID:19749753</ref> CTD of both monomers were measured to be closer together when saturated with Zn<sup>2+</sup>. | This is possible because of the flexible loop that links the TMD and the CTD. This loop harbors the <scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene> which serves as a hinge that allows movement of the CTD. Using [https://en.wikipedia.org/wiki/F%C3%B6rster_resonance_energy_transfer FRET] to measure the distance between the CTD of each monomer fluorescence quenching was observed as the concentration Zn<sup>2+</sup> increased, which supports that idea that Zn<sup>2+</sup> induces a stabilizing conformation change in the CTD.<ref>PMID:19749753</ref> CTD of both monomers were measured to be closer together when saturated with Zn<sup>2+</sup>. | ||
Revision as of 01:15, 20 April 2017
Introduction
Zinc transporter (TC# 2.A.4.7.1) is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological realm, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which are necessary for different biological functions but can prove to be fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response in larger organisms. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researchers better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||