We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1072
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
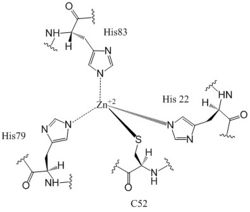
DgcZ is a tetrameric protein from ''E. coli'' made of <scene name='69/694239/Dgcz_ggeef_dom_and_czb_dom/1'>two domains</scene>. Each domain is a dimer, so the whole protein can be called a dimer of dimers. The DgcZ protein has <scene name='69/694239/C2_symmetry/6'>C2</scene> symmetry down its central axis. The catalytic glycine-glycine-glutamate-glutamate-phenylalanine (GGEEF) domain is responsible for synthesizing c-di-GMP, and the regulatory chemoreceptor zinc binding (CZB) domain houses two zinc binding sites. DgcZ binds zinc in the CZB domain with sub-femtomolar (10<sup>-16</sup>M) affinity. When zinc is bound, the CZB and GGEEF domains adopt conformations that inhibit DgcZ function <sup>[1]</sup>. Enzyme DgcZ was co-crystallized with Zinc fixing the structure in its inactivate conformation. The CZB domain is common to many bacterial lineages, including its prevalence in DgcZ homologs. The domain has an important role in signal transduction of bacteria. CZB and GGEEF domains are prevalent in many bacterial proteins from differing strands of ''E. coli'' <sup>[2]</sup>. The GGEEF domain is catalytic in that it contains the active sites used for cyclizing GTP into c-di-GMP. The CZB domain is used for ligand-mediated regulation of c-di-GMP production. | DgcZ is a tetrameric protein from ''E. coli'' made of <scene name='69/694239/Dgcz_ggeef_dom_and_czb_dom/1'>two domains</scene>. Each domain is a dimer, so the whole protein can be called a dimer of dimers. The DgcZ protein has <scene name='69/694239/C2_symmetry/6'>C2</scene> symmetry down its central axis. The catalytic glycine-glycine-glutamate-glutamate-phenylalanine (GGEEF) domain is responsible for synthesizing c-di-GMP, and the regulatory chemoreceptor zinc binding (CZB) domain houses two zinc binding sites. DgcZ binds zinc in the CZB domain with sub-femtomolar (10<sup>-16</sup>M) affinity. When zinc is bound, the CZB and GGEEF domains adopt conformations that inhibit DgcZ function <sup>[1]</sup>. Enzyme DgcZ was co-crystallized with Zinc fixing the structure in its inactivate conformation. The CZB domain is common to many bacterial lineages, including its prevalence in DgcZ homologs. The domain has an important role in signal transduction of bacteria. CZB and GGEEF domains are prevalent in many bacterial proteins from differing strands of ''E. coli'' <sup>[2]</sup>. The GGEEF domain is catalytic in that it contains the active sites used for cyclizing GTP into c-di-GMP. The CZB domain is used for ligand-mediated regulation of c-di-GMP production. | ||
| - | ==Catalytic GGEEF Domain | + | ==Catalytic GGEEF Domain== |
The <scene name='69/694239/Ggeef_domain_zmout_dgcz/2'>GGEEF domain</scene> of DgcZ is part of the GGDEF family of proteins that includes a conserved sequence, GG[DE][DE]F<sup>[3]</sup>.The GGEEF domain is a homodimer consisting of a central five-stranded β-sheet surrounded by five α-helices. The GGEEF domain contains two catalytic half sites that, when combined together in a productive conformation, form the entire <scene name='69/694239/Ggeef_domain_dgcz/6'>active site</scene>. Residues Gly-206, Gly-207, Glu-208, Glu-209, and Phe-210 form the active site. Each <scene name='69/694239/Ggeef_domain_half_site_dgcz/3'>half-site</scene> binds one GTP molecule. DgcZ binds the guanine base of GTP through hydrogen bonds to <scene name='69/694239/Gtp_guanine_bonds_asn_asp_dgcz/6'>Asn 173 and Asp 182</scene>. This scene depicts an inactive form of the protein because it was crystallized with zinc bound. The ribose of each guanosine triphosphate, and subsequent product c-di-GMP riboses, are held only loosely by the enzyme, while the phosphate groups are not bound to the active site at all<sup>[1]</sup>. | The <scene name='69/694239/Ggeef_domain_zmout_dgcz/2'>GGEEF domain</scene> of DgcZ is part of the GGDEF family of proteins that includes a conserved sequence, GG[DE][DE]F<sup>[3]</sup>.The GGEEF domain is a homodimer consisting of a central five-stranded β-sheet surrounded by five α-helices. The GGEEF domain contains two catalytic half sites that, when combined together in a productive conformation, form the entire <scene name='69/694239/Ggeef_domain_dgcz/6'>active site</scene>. Residues Gly-206, Gly-207, Glu-208, Glu-209, and Phe-210 form the active site. Each <scene name='69/694239/Ggeef_domain_half_site_dgcz/3'>half-site</scene> binds one GTP molecule. DgcZ binds the guanine base of GTP through hydrogen bonds to <scene name='69/694239/Gtp_guanine_bonds_asn_asp_dgcz/6'>Asn 173 and Asp 182</scene>. This scene depicts an inactive form of the protein because it was crystallized with zinc bound. The ribose of each guanosine triphosphate, and subsequent product c-di-GMP riboses, are held only loosely by the enzyme, while the phosphate groups are not bound to the active site at all<sup>[1]</sup>. | ||
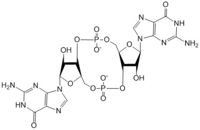
The alpha phosphate is available for attack by the 3 prime hydroxyl group on another GTP. A <scene name='69/694239/Gtp_magnesium_cofactors_dgcz/2'>Magnesium ion</scene> (Mg<sup>2+</sup>) stabilizes the negative charges on the phosphate groups. When in the productive conformation, each GTP is held in close proximity with the α-phosphate groups overlapping C3 of the ribose ring. This conformation allows the α-phospate of one GTP to react with the alcohol group attached to C3 of the ribose on the second GTP, resulting in a cyclization of the two molecules into c-di-GMP. | The alpha phosphate is available for attack by the 3 prime hydroxyl group on another GTP. A <scene name='69/694239/Gtp_magnesium_cofactors_dgcz/2'>Magnesium ion</scene> (Mg<sup>2+</sup>) stabilizes the negative charges on the phosphate groups. When in the productive conformation, each GTP is held in close proximity with the α-phosphate groups overlapping C3 of the ribose ring. This conformation allows the α-phospate of one GTP to react with the alcohol group attached to C3 of the ribose on the second GTP, resulting in a cyclization of the two molecules into c-di-GMP. | ||
Revision as of 15:42, 21 April 2017
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Diguanylate Cyclase DgcZ from Escherichia coli
| |||||||||||