We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1072
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
The <scene name='69/694239/Zb_domain_residues_19-90/7'>CZB domain</scene>, residues 19-90, is responsible for regulating the function of DgcZ due to the presence of two zinc binding sites. The domain contains the <scene name='69/694239/Zinc_binding_domain_zmout/1'>allosteric binding sites</scene> of the enzyme which exhibits cooperative binding. Four residues bind zinc with a high affinity even at 10<sup>-16</sup>M concentrations of Zinc in solution. Due to the tightness of Zinc binding, the complete enzyme has not yet been crystallized in its active conformation without the presence of Zinc metal inhibitor. When zinc is bound, DgcZ activity is limited<sup>[1]</sup>. Two Zinc binding sites are located on the CZB domain. | The <scene name='69/694239/Zb_domain_residues_19-90/7'>CZB domain</scene>, residues 19-90, is responsible for regulating the function of DgcZ due to the presence of two zinc binding sites. The domain contains the <scene name='69/694239/Zinc_binding_domain_zmout/1'>allosteric binding sites</scene> of the enzyme which exhibits cooperative binding. Four residues bind zinc with a high affinity even at 10<sup>-16</sup>M concentrations of Zinc in solution. Due to the tightness of Zinc binding, the complete enzyme has not yet been crystallized in its active conformation without the presence of Zinc metal inhibitor. When zinc is bound, DgcZ activity is limited<sup>[1]</sup>. Two Zinc binding sites are located on the CZB domain. | ||
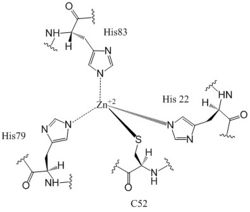
[[Image:Zinc binding site labels.jpg|250 px|left|thumb|'''Figure 5: Zn<sup>+2</sup> Coordination to amino acid residues.''' Three of the four 𝝰 helices of the CZB domain of DgcZ coordinate to the Zn<sup>+2</sup> ion for binding.]] | [[Image:Zinc binding site labels.jpg|250 px|left|thumb|'''Figure 5: Zn<sup>+2</sup> Coordination to amino acid residues.''' Three of the four 𝝰 helices of the CZB domain of DgcZ coordinate to the Zn<sup>+2</sup> ion for binding.]] | ||
| - | Most cells possess efficient Zinc uptake systems, as Zinc is a reactive Lewis Acid. Zinc binds incredibly tightly to this enzyme at subfemtomolar concentrations, attributing to why the enzyme has not been crystallized without Zinc present. The Zinc co-purified with the protein. Zinc allosterically inhibits the activity of enzyme DgcZ through two allosteric binding sites located on the CZB domain <sup>[1]</sup>. The inhibition prevents regulation of GGDEF domain function, the location of the active site. The CZB domain is folded into four anti-parallel α-helices as a 2-fold symmetric homodimer, with the N-terminus on the helix 𝝰4. The allosteric binding site includes a <scene name='69/694239/Zinc_binding_domain_zm_in/ | + | Most cells possess efficient Zinc uptake systems, as Zinc is a reactive Lewis Acid. Zinc binds incredibly tightly to this enzyme at subfemtomolar concentrations, attributing to why the enzyme has not been crystallized without Zinc present. The Zinc co-purified with the protein. Zinc allosterically inhibits the activity of enzyme DgcZ through two allosteric binding sites located on the CZB domain <sup>[1]</sup>. The inhibition prevents regulation of GGDEF domain function, the location of the active site. The CZB domain is folded into four anti-parallel α-helices as a 2-fold symmetric homodimer, with the N-terminus on the helix 𝝰4. The allosteric binding site includes a <scene name='69/694239/Zinc_binding_domain_zm_in/2'>3His/1Cys</scene> motif that uses amino acids H22 of 𝝰1, C52 of 𝝰2, and H79 and H83 of 𝝰3, spanning three of the four alpha helices of the CZB domain and coordinating the Zinc residue in a tetrahedral fashion. For <scene name='69/694239/Ntermctermdgcz/2'>clarification</scene>, the entirety of 𝝰helix 2 on one monomer of CZB is not successfully crystallized after the Cys52 residue and is not the N-terminal residue. |
Zahringer et al. mutated Cys52 to Ala through <span class="plainlinks">[https://en.wikipedia.org/wiki/Site-directed_mutagenesis site-directed mutagenesis]</span>, resulting in a lack of coordination on α2. The cysteine residue is not essential for Zinc binding, as Zinc still coordinates to the three His residues with the Cys52Ala mutation, but α2 is free to move and expose the Zinc binding pocket. This exposure was found to lower the protein's affinity for zinc, as the mutation of cysteine to alanine increased the activity of the DgcZ. When not coordinated to zinc, the CZB domain presumably adopts a conformation that straightens the <scene name='69/694239/Czbd_with_helices_labeled/4'>α2 helix</scene>, shifting <scene name='69/694239/Hydrophobicity_int_residues/3'>hydrophobic residues</scene> on the α-helices into the center and the GGEEF domain into its productive conformation, increasing activity of DgcZ. Until the entire protein is crystallized without zinc, this conformation change is merely hypothesized. Activity increases without Zinc due to activation of poly-GlcNAc production and biofilm formation, and maximal cyclic di-GMP production. | Zahringer et al. mutated Cys52 to Ala through <span class="plainlinks">[https://en.wikipedia.org/wiki/Site-directed_mutagenesis site-directed mutagenesis]</span>, resulting in a lack of coordination on α2. The cysteine residue is not essential for Zinc binding, as Zinc still coordinates to the three His residues with the Cys52Ala mutation, but α2 is free to move and expose the Zinc binding pocket. This exposure was found to lower the protein's affinity for zinc, as the mutation of cysteine to alanine increased the activity of the DgcZ. When not coordinated to zinc, the CZB domain presumably adopts a conformation that straightens the <scene name='69/694239/Czbd_with_helices_labeled/4'>α2 helix</scene>, shifting <scene name='69/694239/Hydrophobicity_int_residues/3'>hydrophobic residues</scene> on the α-helices into the center and the GGEEF domain into its productive conformation, increasing activity of DgcZ. Until the entire protein is crystallized without zinc, this conformation change is merely hypothesized. Activity increases without Zinc due to activation of poly-GlcNAc production and biofilm formation, and maximal cyclic di-GMP production. | ||
Revision as of 17:33, 21 April 2017
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Diguanylate Cyclase DgcZ from Escherichia coli
| |||||||||||
![Figure 3: Diagram of DgcZ DgcZ is shown in its active (left) and inactive (right) conformations. The boxes represent the GGEEF domains of the enzyme, while the cylinders represent the alpha helices of the CZB domain, which contains the Zinc binding sites[3]. Binding Zn+2 inactivates the enzyme. The red vs blue coloring represents the symmetry of the enzyme.](/wiki/images/thumb/d/df/DgcZ_Conformation_change_1.png/250px-DgcZ_Conformation_change_1.png)