Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1069
From Proteopedia
| Line 6: | Line 6: | ||
==Structure== | ==Structure== | ||
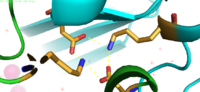
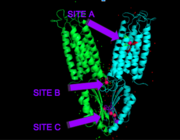
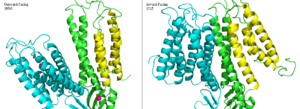
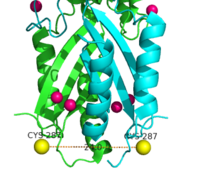
| - | YiiP is a homodimer [https://en.wikipedia.org/wiki/Protein_dimer (protein dimer)], with each monomer consisting of 238 residues with TransMembrane (<scene name='69/694236/Realtmd1/1'>TMD</scene>) and C-Terminal (<scene name='69/694236/Realctd1/1'>CTD</scene>) domains (colored blue) that are connected via a charge interlocking mechanism located on a flexible loop. In total, the Yiip protein has three Zn<sup>2+</sup> <scene name='69/694236/Bindingsiteswcolor/2'>binding sites</scene>, site A, B, and C. Site A is located in the <scene name='69/694236/Bindingsiteswcolor/3'>TMD</scene> of the protein, highlighted in purple, site C is located in the <scene name='69/694236/Bindingsiteswcolor/4'>CTD</scene>, now seen in purple, and site B is located at the junction of the two domains. The TMD, where Zn<sup>2+</sup> binding site A resides, consists of 6 transmembrane (TM) helices, 4 of which, <scene name='69/694236/Tmlabels/1'>TM1,TM2,TM4, and TM5</scene> (labeled on only one monomer, but present on both), pivot about the ion binding site A. The remaining two helices, <scene name='69/694236/Tm3tm6/2'>TM3 and TM6</scene>, are oriented <scene name='69/694236/Tm3tm6antiparallel/1'>antiparallel</scene> to the bundle. Movement of these helices plays a role in the function of Zn<sup>2+</sup> transport. | + | YiiP is a homodimer [https://en.wikipedia.org/wiki/Protein_dimer (protein dimer)], with each monomer consisting of 238 residues with TransMembrane (<scene name='69/694236/Realtmd1/1'>TMD</scene>) and C-Terminal (<scene name='69/694236/Realctd1/1'>CTD</scene>) domains (colored blue) that are connected via a charge interlocking mechanism (<scene name='75/756372/Bestsaltbridgetransparent/1'>salt bridge</scene>) located on a flexible loop. In total, the Yiip protein has three Zn<sup>2+</sup> <scene name='69/694236/Bindingsiteswcolor/2'>binding sites</scene>, site A, B, and C. Site A is located in the <scene name='69/694236/Bindingsiteswcolor/3'>TMD</scene> of the protein, highlighted in purple, site C is located in the <scene name='69/694236/Bindingsiteswcolor/4'>CTD</scene>, now seen in purple, and site B is located at the junction of the two domains. The TMD, where Zn<sup>2+</sup> binding site A resides, consists of 6 transmembrane (TM) helices, 4 of which, <scene name='69/694236/Tmlabels/1'>TM1,TM2,TM4, and TM5</scene> (labeled on only one monomer, but present on both), pivot about the ion binding site A. The remaining two helices, <scene name='69/694236/Tm3tm6/2'>TM3 and TM6</scene>, are oriented <scene name='69/694236/Tm3tm6antiparallel/1'>antiparallel</scene> to the bundle. Movement of these helices plays a role in the function of Zn<sup>2+</sup> transport. |
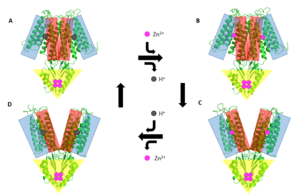
A large portion of the protein containing binding site C, the <scene name='69/694236/Realctd1/1'>CTD</scene>, approximately 30 <scene name='69/694236/Angstrom/2'>Å</scene> in length<sup>[1]</sup>, protrudes into the cytoplasm functioning as a Zn<sup>2+</sup> sensor within the cell. Zn<sup>2+</sup> binding at site C helps hold the CTD together and is thought to stabilize conformational changes in YiiP. YiiP has two different functional conformations which dictates whether or not YiiP is open to the periplasm or the cytoplasm. This [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridge] acts as the hinge for Yiip's conformational changes. | A large portion of the protein containing binding site C, the <scene name='69/694236/Realctd1/1'>CTD</scene>, approximately 30 <scene name='69/694236/Angstrom/2'>Å</scene> in length<sup>[1]</sup>, protrudes into the cytoplasm functioning as a Zn<sup>2+</sup> sensor within the cell. Zn<sup>2+</sup> binding at site C helps hold the CTD together and is thought to stabilize conformational changes in YiiP. YiiP has two different functional conformations which dictates whether or not YiiP is open to the periplasm or the cytoplasm. This [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) salt bridge] acts as the hinge for Yiip's conformational changes. | ||
Revision as of 18:08, 21 April 2017
Introduction
Zinc transporter (TC# 2.A.4.7.1) is an integral membrane protein found in the membrane of Esherichia coli and a member of the cation diffusion facilitator family. Members of this family occur all throughout the biological realm, their primary function being the export of divalent transition metal ions from the cytoplasm to the extracellular space [1]. They work to regulate the amount of divalent metals inside of the cell, which are necessary for different biological functions but can prove to be fatal to the cell in excess amounts. Zinc is essential for the growth and development of cells and zinc levels can affect everything from gene expression to immune response in larger organisms. While YiiP is an integral membrane protein in the cells of Escherichia coli, understanding the mechanism of regulation behind it can help researchers better understand the cation diffusion facilitator equivalents in eukaryotic cells.
| |||||||||||