We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Luke Edward Severinac/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
=='''Zymogen'''== | =='''Zymogen'''== | ||
| - | In addition to a self-cleavage mechanism, Caspase-6 <scene name='75/752344/Caspase-6_zymogen_yeahboi/1'>zymogen</scene> can be activated through cleavage by Caspase-3, as well as other enzymes. This activation by cleavage is highly conserved across the caspase family, but activation through self-cleavage is uniquely recognized as the primary mechanism for Caspase-6 activation. In this self-cleavage mechanism, cleavage must occur at <scene name='75/752344/Caspase-6_cleavage_sites_real/1'>three sites</scene> in order to remove the <scene name='75/752344/Caspase-6_prodomain/1'>pro-domain</scene> located at the N-terminus and the <scene name='75/752344/Caspase-6_intersubunit_linker/1'>intersubunit linker</scene> located within the protein. These cleavages are both sequence specific and ordered, starting with cleavage of the pro-domain at <scene name='75/752344/Caspase-6_prodomain_cleavage/1'>residue 30</scene>. Removal of the intersubunit linker then occurs through cleavage at two sites, <scene name='75/752344/Caspase-6_176-179_cleavageyis/1'>DVVD179 and TEVD193</scene><ref name="RegMechStructure">PMID: 24419379 </ref>. It has been proposed that this sequence of cleavage is due to the pro-domain being more readily available to enter the active site, whose presence inhibits Caspase-6's ability to cleave the intersubunit loop and self-activate; The prodomain acts as a “suicide protector”, preventing the TEVD193 cleavage site from the active site | + | In addition to a self-cleavage mechanism, Caspase-6 <scene name='75/752344/Caspase-6_zymogen_yeahboi/1'>zymogen</scene> can be activated through cleavage by Caspase-3, as well as other enzymes. This activation by cleavage is highly conserved across the caspase family, but activation through self-cleavage is uniquely recognized as the primary mechanism for Caspase-6 activation. In this self-cleavage mechanism, cleavage must occur at <scene name='75/752344/Caspase-6_cleavage_sites_real/1'>three sites</scene> in order to remove the <scene name='75/752344/Caspase-6_prodomain/1'>pro-domain</scene> located at the N-terminus and the <scene name='75/752344/Caspase-6_intersubunit_linker/1'>intersubunit linker</scene> located within the protein. These cleavages are both sequence specific and ordered, starting with cleavage of the pro-domain at <scene name='75/752344/Caspase-6_prodomain_cleavage/1'>residue 30</scene>. Removal of the intersubunit linker then occurs through cleavage at two sites, <scene name='75/752344/Caspase-6_176-179_cleavageyis/1'>DVVD179 and TEVD193</scene><ref name="RegMechStructure">PMID: 24419379 </ref>. It has been proposed that this sequence of cleavage is due to the pro-domain being more readily available to enter the active site, whose presence inhibits Caspase-6's ability to cleave the intersubunit loop and self-activate; The prodomain acts as a “suicide protector”, preventing the TEVD193 cleavage site from the active site<ref name="ActRegofCas6inND">PMID: 25340928 </ref>. After both cleavages occur, <scene name='75/752344/Active_caspase_6_dimer/1'>active Caspase-6</scene> remains in solution as a dimer. |
=='''Active State'''== | =='''Active State'''== | ||
| Line 12: | Line 12: | ||
yellow - ligand | yellow - ligand | ||
red - generic surface]] | red - generic surface]] | ||
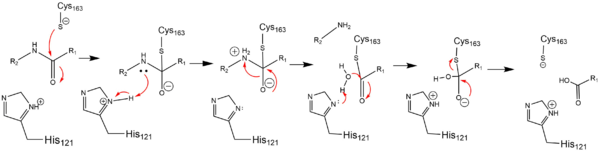
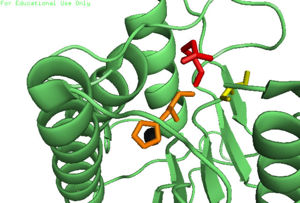
| - | In order to function as an endopeptidase, each <scene name='75/752344/Active_caspase_6_monomer/1'>monomer</scene | + | In order to function as an endopeptidase, each <scene name='75/752344/Active_caspase_6_monomer/1'>monomer</scene> of active Caspase-6 utilizes a <scene name='75/752344/Catalytic_triad_real/1'>catalytic triad</scene> composed of <scene name='75/752344/Catalytic_his-121_monomer/1'>His-121</scene>, <scene name='75/752344/Catalytic_glu-123_monomer/1'>Glu-123</scene>, and <scene name='75/752344/Catalytic_cys-163_monomer/1'>Cys-163</scene> to cleave polypeptide ligands that can include neuronal proteins and [https://en.wikipedia.org/wiki/Tubulin tubulins]<ref name="ActiveStateCrys">PMID: 21917678 </ref>. In the theorized mechanism, atoms are shown in their resting ionic states; His-121 acts as an acid catalyst, Glu-123 acts as a base catalyst to deprotonate Cys-163, which then acts as covalent catalyst. |
[[Image:Cystine Aspartase.png|600 px|active site mechanism]] | [[Image:Cystine Aspartase.png|600 px|active site mechanism]] | ||
=='''Zinc Inhibition'''== | =='''Zinc Inhibition'''== | ||
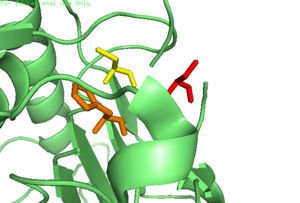
| - | Caspase-6 can also assume an inactive state, which exists as a <scene name='75/752344/Casp_6_inactive_dimer/1'>dimer</scene> in its biological unit. For each <scene name='75/752344/Casp_6_inactive_monomer/1'>monomer</scene>, Caspase-6 function is primarily inhibited by the binding of a <scene name='75/752344/Casp_6_inactive_monomer_zinc/1'>zinc</scene> ion, which binds to an <scene name='75/752344/Casp_6_alosteric_site/1'>allosteric site</scene> instead of the <scene name='75/752344/Casp_6_alostericactive_site/1'>active site</scene>. This allosteric site is located on the opposite side of the protein relative to the active site. The zinc ion is bound to <scene name='75/752344/Caspase6_alloster_resid/1'>three residues</scene>, Lys-36, Glu-244, and His-287. Once the ion is bound to the protein, it is then stabilized by a <scene name='75/752344/Caspase6_alloster_h20/1'>water molecule</scene> found in the cytoplasm. The binding of zinc at the exosite is suggested to cause a conformational change in the protein from an <scene name='75/752344/Catalytic_triad_real_monomer/1'>active state</scene> to an <scene name='75/752344/Casp_6_inactive_cat_triad/1'>inactive state</scene> that misaligns catalytic residues and inhibits activity of the enzyme. It has been proposed that helices of the active dimer must rotate or move in some other way to provide these ideal interactions with zinc. This subtle shift is most likely the cause for allosteric inhibition. As the helices move to bind zinc, the amino acids of the active site become misaligned. The altered positions of the amino acids no longer provide ideal interactions for incoming substrates. After zinc binds, substrates may still enter the active site, but no catalytic activity will occur. | + | Caspase-6 can also assume an inactive state, which exists as a <scene name='75/752344/Casp_6_inactive_dimer/1'>dimer</scene> in its biological unit. For each <scene name='75/752344/Casp_6_inactive_monomer/1'>monomer</scene>, Caspase-6 function is primarily inhibited by the binding of a <scene name='75/752344/Casp_6_inactive_monomer_zinc/1'>zinc</scene> ion, which binds to an <scene name='75/752344/Casp_6_alosteric_site/1'>allosteric site</scene> instead of the <scene name='75/752344/Casp_6_alostericactive_site/1'>active site</scene>. This allosteric site is located on the opposite side of the protein relative to the active site. The zinc ion is bound to <scene name='75/752344/Caspase6_alloster_resid/1'>three residues</scene>, Lys-36, Glu-244, and His-287. Once the ion is bound to the protein, it is then stabilized by a <scene name='75/752344/Caspase6_alloster_h20/1'>water molecule</scene> found in the cytoplasm. The binding of zinc at the exosite is suggested to cause a conformational change in the protein from an <scene name='75/752344/Catalytic_triad_real_monomer/1'>active state</scene> to an <scene name='75/752344/Casp_6_inactive_cat_triad/1'>inactive state</scene> that misaligns catalytic residues and inhibits activity of the enzyme. It has been proposed that helices of the active dimer must rotate or move in some other way to provide these ideal interactions with zinc. This subtle shift is most likely the cause for allosteric inhibition<ref name="zincmedallinhib">PMID: 22891250 </ref>. As the helices move to bind zinc, the amino acids of the active site become misaligned. The altered positions of the amino acids no longer provide ideal interactions for incoming substrates. After zinc binds, substrates may still enter the active site, but no catalytic activity will occur. |
[[Image:4FXO-FINAL.jpg|300px|Caspase-6 w/ Zinc Bound]] [[Image:3s70.jpg|300px|Caspase-6 w/o Zinc Bound]] | [[Image:4FXO-FINAL.jpg|300px|Caspase-6 w/ Zinc Bound]] [[Image:3s70.jpg|300px|Caspase-6 w/o Zinc Bound]] | ||
=='''Phosphorylation'''== | =='''Phosphorylation'''== | ||
| - | The function of Caspase-6 can be inhibited by phosphorylation of Ser-257. The exact mechanism of this reaction remains unidentified at the time of publication, but proceeds when ARK5 kinase is present. This modification can occur before and after zymogen activation. The phosphoryl group inhibits Caspase-6 through steric interference. When Ser-257 is phosphorylated, the amino acid residue interacts with <scene name='75/752344/Caspase-6_his-208/1'>Pro-201</scene>, causing a shift in the helices of Caspase-6. This is shown in the <scene name='75/752344/Caspase-6_s257d_mutant/1'>S257D Caspase-6 mutant</scene>, whose mutation mimics phosphorylation<ref name="Phosregcasp6subsbindgroove">PMID: 22483120 </ref>. The shift misaligns and disrupts residues found in the active site. This conformational difference prevents the intersubunit linker from entering during zymogen activation and the self-cleaved active dimer cannot be formed. Additionally, no new substrate is able to enter the active site. | + | The function of Caspase-6 can be inhibited by phosphorylation of Ser-257. The exact mechanism of this reaction remains unidentified at the time of publication, but proceeds when ARK5 kinase is present. This modification can occur before and after zymogen activation. The phosphoryl group inhibits Caspase-6 through steric interference. When Ser-257 is phosphorylated, the amino acid residue interacts with <scene name='75/752344/Caspase-6_his-208/1'>Pro-201</scene>, causing a shift in the helices of Caspase-6<ref name="ActRegofCas6inND">PMID: 25340928 </ref>. This is shown in the <scene name='75/752344/Caspase-6_s257d_mutant/1'>S257D Caspase-6 mutant</scene>, whose mutation mimics phosphorylation<ref name="Phosregcasp6subsbindgroove">PMID: 22483120 </ref>. The shift misaligns and disrupts residues found in the active site. This conformational difference prevents the intersubunit linker from entering during zymogen activation and the self-cleaved active dimer cannot be formed. Additionally, no new substrate is able to enter the active site. |
=='''Medical Relevance'''== | =='''Medical Relevance'''== | ||
| Line 29: | Line 29: | ||
=='''References'''== | =='''References'''== | ||
{{reflist}} | {{reflist}} | ||
| - | 4IYR 24419379 zymogen | ||
Revision as of 02:34, 23 April 2017
Caspase-6 in Homo sapiens
| |||||||||||