Sandbox Reserved 1236
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
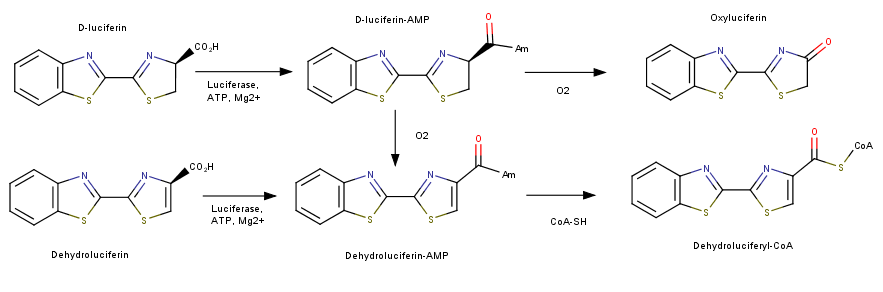
Luciferase catalyzes two reactions; both reactions use an adenylation reaction along with ATP. The bioluminescent pathway produces Oxyluciferin and a photon of light. The other pathways does not produce light and is known as the nonluminescent reaction. This mechanism utilizes luciferin and produces Luciferyl-CoA. This addiotion of a CoA has been shown to be related to Acyl-CoA synthase used in the activation of fatty acids for oxidation. | Luciferase catalyzes two reactions; both reactions use an adenylation reaction along with ATP. The bioluminescent pathway produces Oxyluciferin and a photon of light. The other pathways does not produce light and is known as the nonluminescent reaction. This mechanism utilizes luciferin and produces Luciferyl-CoA. This addiotion of a CoA has been shown to be related to Acyl-CoA synthase used in the activation of fatty acids for oxidation. | ||
| + | |||
| + | [[Image:luciferase.png]] | ||
| + | This image depict the two reactions that luciferase can catalyze. The first being the bioluminencent pathway producing a photon of light and the second, following a mechanism similar to fatty acid synthase, producing a Dehydroluciferyl-CoA. <ref>Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.</ref> | ||
==Origin== | ==Origin== | ||
| Line 21: | Line 24: | ||
The structure of this protein comprises of two prominent domains. The larger one contains an N terminal distorted beta-barrel accompanied by alpha helices. The second and smaller unit is consist of a beta sheet and alpha helix complex <ref>Viviani, V. R. (2002). The origin, diversity, and structure function relationships of insect luciferases. Cellular and Molecular Life Sciences, 59(11), 1833-1850. </ref>. The process of fluorescence is achieved through a two-step oxidation reaction involving the substrate Lucinferin accompanied with ATP, Magnesium and oxygen. The first step consist of using ATP-Mg in an Acylation reaction of the COOH group on Lucinferin producing a Luciferyl adenylate intermediate and a phosphate group. The second reaction uses oxygen to create an excited state of the molecule. The molecule then returns to its ground state emitting a photon of light (Conti et al., 1996). | The structure of this protein comprises of two prominent domains. The larger one contains an N terminal distorted beta-barrel accompanied by alpha helices. The second and smaller unit is consist of a beta sheet and alpha helix complex <ref>Viviani, V. R. (2002). The origin, diversity, and structure function relationships of insect luciferases. Cellular and Molecular Life Sciences, 59(11), 1833-1850. </ref>. The process of fluorescence is achieved through a two-step oxidation reaction involving the substrate Lucinferin accompanied with ATP, Magnesium and oxygen. The first step consist of using ATP-Mg in an Acylation reaction of the COOH group on Lucinferin producing a Luciferyl adenylate intermediate and a phosphate group. The second reaction uses oxygen to create an excited state of the molecule. The molecule then returns to its ground state emitting a photon of light (Conti et al., 1996). | ||
| - | + | ||
A single peptide has been discover that plays a vital role in the photooxidation by Luciferase. The specific amino acid is a histidine located in the region <scene name='75/750285/Luciferasemonomer114/2'>244HHGF247</scene> of the protein <ref>Branchini, B. R., Magyar, R. A., Marcantonio, K. M., Newberry, K. J., Stroh, J. G., Hinz, L. K., & Murtiashaw, M. H. (1997). Identification of a Firefly Luciferase Active Site Peptide Using a Benzophenone-based Photooxidation Reagent. Journal of Biological Chemistry, 272(31), 19359-19364.</ref>. It has been shown to be necessary for the use of oxygen in the second part of the reaction. | A single peptide has been discover that plays a vital role in the photooxidation by Luciferase. The specific amino acid is a histidine located in the region <scene name='75/750285/Luciferasemonomer114/2'>244HHGF247</scene> of the protein <ref>Branchini, B. R., Magyar, R. A., Marcantonio, K. M., Newberry, K. J., Stroh, J. G., Hinz, L. K., & Murtiashaw, M. H. (1997). Identification of a Firefly Luciferase Active Site Peptide Using a Benzophenone-based Photooxidation Reagent. Journal of Biological Chemistry, 272(31), 19359-19364.</ref>. It has been shown to be necessary for the use of oxygen in the second part of the reaction. | ||
| - | ==Structure Specifications== | ||
| + | ==Structure Specifications== | ||
| + | The attractive feature for the study and application of luciferase is its bioluminescent activity. The color of light produced has been found to vary depending on the organism that the protein is retrieved from. The fluorsence of light ranges through various wavelengths of light some of which include red, green and yellow light. This difference has a connection to the specificity of the active site of luciferase. Depending on the source of the protein different residues will produce a certain color of fluorescence. Variations in luciferase extracted from japanese fire flies has revealed this specificity. In high energy analogs of luciferase reveal the 286th residue is important in determine the color of light. When comparing green fluorescent japanese luciferin the active site contain an Ser286, while the red emitting variant has an Asp286. These findings indicate that slight variations in the active sight of luciferase can produce various effects on its bioluminencent activity. <ref>Goodsell, David . "Molecule of the Month: Luciferase." PDB-101: Luciferase. RSCB Protein Data Bank, June 2006. Web. 28 Apr. 2017.</ref> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 22:04, 28 April 2017
| This Sandbox is Reserved from Jan 17 through June 31, 2017 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, USA. This reservation includes Sandbox Reserved 1225 through Sandbox Reserved 1244. |
To get started:
More help: Help:Editing |
Luciferase
| |||||||||||
References
- ↑ Conti, E., Franks, N. P., & Brick, P. (1996). Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure, 4(3), 287-298.
- ↑ Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.
- ↑ Khurana, Pankaj, Rajesh S. Gokhale, and Debasisa Mohanty. "Genome scale prediction of substrate specificity for acyl adenylate superfamily of enzymes based on active site residue profiles." BMC Bioinformatics 11.1 (2010): 57. ResearchGate. Web. 28 Mar. 2017.
- ↑ Wet, J. R., Wood, K. V., Helinski, D. R., & Deluca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences, 82(23), 7870-7873.
- ↑ Viviani, V. R. (2002). The origin, diversity, and structure function relationships of insect luciferases. Cellular and Molecular Life Sciences, 59(11), 1833-1850.
- ↑ Branchini, B. R., Magyar, R. A., Marcantonio, K. M., Newberry, K. J., Stroh, J. G., Hinz, L. K., & Murtiashaw, M. H. (1997). Identification of a Firefly Luciferase Active Site Peptide Using a Benzophenone-based Photooxidation Reagent. Journal of Biological Chemistry, 272(31), 19359-19364.
- ↑ Goodsell, David . "Molecule of the Month: Luciferase." PDB-101: Luciferase. RSCB Protein Data Bank, June 2006. Web. 28 Apr. 2017.