We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1235
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== '''Function''' == | == '''Function''' == | ||
| - | The function of this protein allows fish to inhibit the process of recrystalization of water as fishes swim to colder climates , for example like the winter flounder migrating to the northern pole.The protein is secreted by the liver into the blood. Although recently research has shown that the Antifreeze protein isoforms are produced near areas like the skin, scales, fin, or gills in order to become a defense response to the ice propagation.<ref> | + | The function of this protein allows fish to inhibit the process of recrystalization of water as fishes swim to colder climates , for example like the winter flounder migrating to the northern pole.The protein is secreted by the liver into the blood. Although recently research has shown that the Antifreeze protein isoforms are produced near areas like the skin, scales, fin, or gills in order to become a defense response to the ice propagation.<ref>Fletcher, Garth L., Choy L. Hew, and Peter L. Davies. "Antifreeze Proteins of Teleost Fishes." Antifreeze Proteins of Teleost Fishes. Annual Reviews, 1 Mar. 2001. Web. 03 Apr. 2017.</ref> The molecule is found in many other fish as well that migrate to colder climates such as the shorthorn sculpin, and longhorn sculpin. The function is to depress freezing temperature in order to stop the process of recrystallization to occur.<ref>Fletcher, G. L., C. L. Hew, and P. L. Davies. "Antifreeze Proteins of Teleost Fishes." Annual Review of Physiology. U.S. National Library of Medicine, 2001. Web. 03 May 2017.</ref> recrystallization is When water begins to freeze, many small crystals form, but then a few small crystals dominate and grow larger and larger, forcing the water molecules from the surrounding small crystals to bond with the crystals. Thus making it manageable for fishes like the winter flounder to swim in colder climates.<ref>Goodsell, David. "Antifreeze Proteins." PDB-101: Antifreeze Proteins. N.p., Dec. 2009. Web. 03 May 2017.</ref> |
| Line 28: | Line 28: | ||
[[Image:Picture 1 by me.jpg]] | [[Image:Picture 1 by me.jpg]] | ||
| - | [[Image:Anti_freeze_protein_1_pciture_3.png]]<ref> | + | [[Image:Anti_freeze_protein_1_pciture_3.png]]<ref>"ANTI-FREEZE PROTEIN (AFP)." Team:KULeuven/Afp. N.p., n.d. Web. 03 May 2017.</ref> |
| - | [[Image:Anti_freeze_protein_1_pciture_2.png]]<ref> | + | [[Image:Anti_freeze_protein_1_pciture_2.png]]<ref>Patel, Shivang. "Biotechnology." Antifreeze Proteins. N.p., 01 Jan. 1970. Web. 03 May 2017.</ref> |
== '''Mechanism''' == | == '''Mechanism''' == | ||
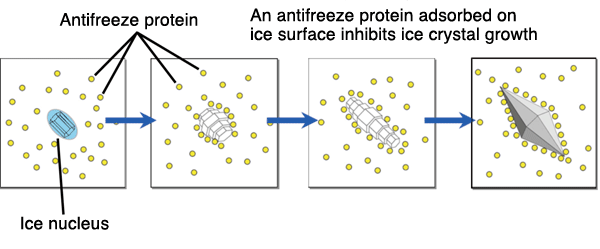
| - | Thermal hysteresis, the separation of the melting and freezing points, is obtained in the mechanism of hyperactive anti-freeze protein 1 very easily. The Anti-freeze proteins are adsorbed onto the ice crystals when the temperatures are reaching around the freezing point and are melted off the ice crystal when the melting point is achieved. The adsorbing is irreversible due to bond strength and can only become irreversible due to a thermal hysteresis of melting point. When the ice tries to grow with the antifreeze proteins they grow in convex surfaces and this will make the ice crystal's expansion go to a lower freezing point. Thus, preventing more freezing to occur.<ref> | + | Thermal hysteresis, the separation of the melting and freezing points, is obtained in the mechanism of hyperactive anti-freeze protein 1 very easily. The Anti-freeze proteins are adsorbed onto the ice crystals when the temperatures are reaching around the freezing point and are melted off the ice crystal when the melting point is achieved. The adsorbing is irreversible due to bond strength and can only become irreversible due to a thermal hysteresis of melting point. When the ice tries to grow with the antifreeze proteins they grow in convex surfaces and this will make the ice crystal's expansion go to a lower freezing point. Thus, preventing more freezing to occur.<ref>Kristiansen, E., and K. E. Zachariassen. "The Mechanism by Which Fish Antifreeze Proteins Cause Thermal Hysteresis." Cryobiology. U.S. National Library of Medicine, Dec. 2005. Web. 03 May 2017.</ref> |
== '''Disease/Mutations''' == | == '''Disease/Mutations''' == | ||
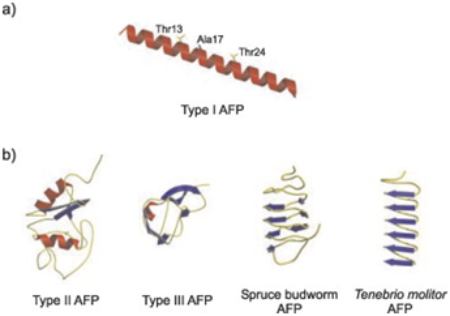
| - | Mutations were studied by replacing the Thr residue of the alpha helix with Valine which in the end still preserved some activity of the hydrogen bonding needed for the antifreeze activity. Another mutation was made to reside adjacent to the Thr-rich face, which was the Ala17 residue. The Ala17 was replaced with Leucine, and this led to the ceased functionality of all antifreeze activity. This this led to the researchers to think that the ice binding face of the Antifreeze protein would most likely need Ala17, Ala21,Thr13, and all equivalent residues at the 11-amino acid intervals.<ref> | + | Mutations were studied by replacing the Thr residue of the alpha helix with Valine which in the end still preserved some activity of the hydrogen bonding needed for the antifreeze activity. Another mutation was made to reside adjacent to the Thr-rich face, which was the Ala17 residue. The Ala17 was replaced with Leucine, and this led to the ceased functionality of all antifreeze activity. This this led to the researchers to think that the ice binding face of the Antifreeze protein would most likely need Ala17, Ala21,Thr13, and all equivalent residues at the 11-amino acid intervals.<ref>Graether, Steffen P., Carolyn M. Slupsky, Peter L. Daviews, and Brain D. Sykes. "Structure of Type I Antifreeze Protein and Mutants in Supercooled Water." Biophysical Journal 81 (2001): 1677-683. Https://www.ncbi.nlm.nih.gov. Web.</ref> |
== '''Relevance''' == | == '''Relevance''' == | ||
| - | The relevance of Antifreeze protein 1 is allowing fish to avoid freezing through halting the growth of the ice and lowering the freezing point.<ref> | + | The relevance of Antifreeze protein 1 is allowing fish to avoid freezing through halting the growth of the ice and lowering the freezing point.<ref>Fletcher, Garth L., Choy L. Hew, and Peter L. Davies. "Antifreeze Proteins of Teleost Fishes." Antifreeze Proteins of Teleost Fishes | Annual Review of Physiology. N.p., n.d. Web. 03 May 2017.</ref> The evidence of convergent evolution is shown through the various forms of Antifreeze proteins. There are proteins that have the same function. All of these are small proteins with threonine and to binds to the ice crystal . These proteins are used in organisms from pout, white flounder, insects, yellow mealworm beetle, spruce budworm moth, and the snow fleas. |
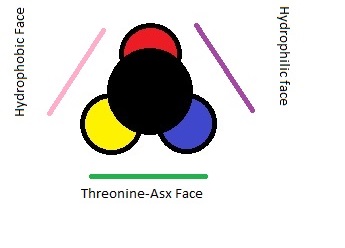
=='''Structure''' == | =='''Structure''' == | ||
| - | This version of AFP could be recorded as the best documented, for it is the first to have three-dimensional structure determined. it is about 3.3 to 4.5 k Daltons in size. its structure consists of single, long, amphipathic alpha helix. The three dimensional structure consists of three faces: a hydrophobic face, a hydrophilic face, and a Thr-Asx face <ref> | + | This version of AFP could be recorded as the best documented, for it is the first to have three-dimensional structure determined. it is about 3.3 to 4.5 k Daltons in size. its structure consists of single, long, amphipathic alpha helix. The three dimensional structure consists of three faces: a hydrophobic face, a hydrophilic face, and a Thr-Asx face <ref>And, P. L Davies. "P L Davies." The FASEB Journal. N.p., 01 May 1990. Web. 03 May 2017.</ref> |
Current revision
| This Sandbox is Reserved from Jan 17 through June 31, 2017 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, USA. This reservation includes Sandbox Reserved 1225 through Sandbox Reserved 1244. |
To get started:
More help: Help:Editing |
Antifreeze protein 1
| |||||||||||