Sandbox Reserved 1239
From Proteopedia
| Line 10: | Line 10: | ||
[[Image:IMG_5804.jpg]] | [[Image:IMG_5804.jpg]] | ||
| - | == ''' | + | == '''Regulation''' == |
| + | As mentioned before, occludin is essential in tight junctions between cells. However, it alone is not sufficient to create a tight junction. Occludin associates with two claudin proteins, which together have been found to be essential to forming a tight junction strand. Other studies found that ZO-1 and occludin together mediate cell adhesion, indicating the pivotal role occludin plays in cell-cell adhesion and tight junctions [6]. | ||
| + | It has been found that occluding expression can be regulated by posttranscriptional methods such as gene splicing. Gene splicing also leads to an isoform of occludin that extends the amino terminus by 56 amino acids [2]. Experimental regulation of occludin has helped reveal and confirm possible functions. When the sequence of one of the extracellular loops of occludin was neutralized and disrupted, cell-cell adhesion was interfered with [6]. | ||
| + | Occludin is also subject to posttranslational regulation. Proteolytic cleavage of occludin leads to barrier disruption, which often leads to disease and restructure of tissue framework. Occludin can be regulated by reversible phosphorylation. Phosphorylation of key serine, tyrosine, and threonine occludin residues is essential in tight junction construction [2]. Occludin phosphorylation corresponds with vessel barrier dysfunction [7]. | ||
| + | |||
| + | == '''Disease''' == | ||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 18:31, 5 May 2017
| This Sandbox is Reserved from Jan 17 through June 31, 2017 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, USA. This reservation includes Sandbox Reserved 1225 through Sandbox Reserved 1244. |
To get started:
More help: Help:Editing |
Contents |
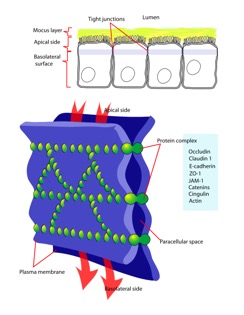
Function and Location
Occludin is one of the proteins absolutely necessary in maintaining tight junctions of cells. Tight junctions preserve the integrity of polarized cells, allowing homeostasis to continue. Polarized cells can be broadly defined as cells that have two dynamically different faces for functionally different purposes. Each face is different in protein and membrane composition. For example, intestinal cells have an apical surface and basolateral surface. The apical surface faces the lumen and intakes material, while the basolateral surface interacts with blood vessels. Proteins and lipids can diffuse laterally and freely through a membrane [1]. However, in cells that are meant to be polarized, it would be catastrophic to have proteins exchanged between apical and basolateral surfaces due to their pivotal roles in homeostasis. Tight junctions restrict lateral diffusion, keeping cell surfaces polarized [2]. Tight junctions, and therefore occluding proteins, heavily influence cell-cell interactions and signal transduction. Cell-cell interactions regulate gene expression and cell differentiation [5].
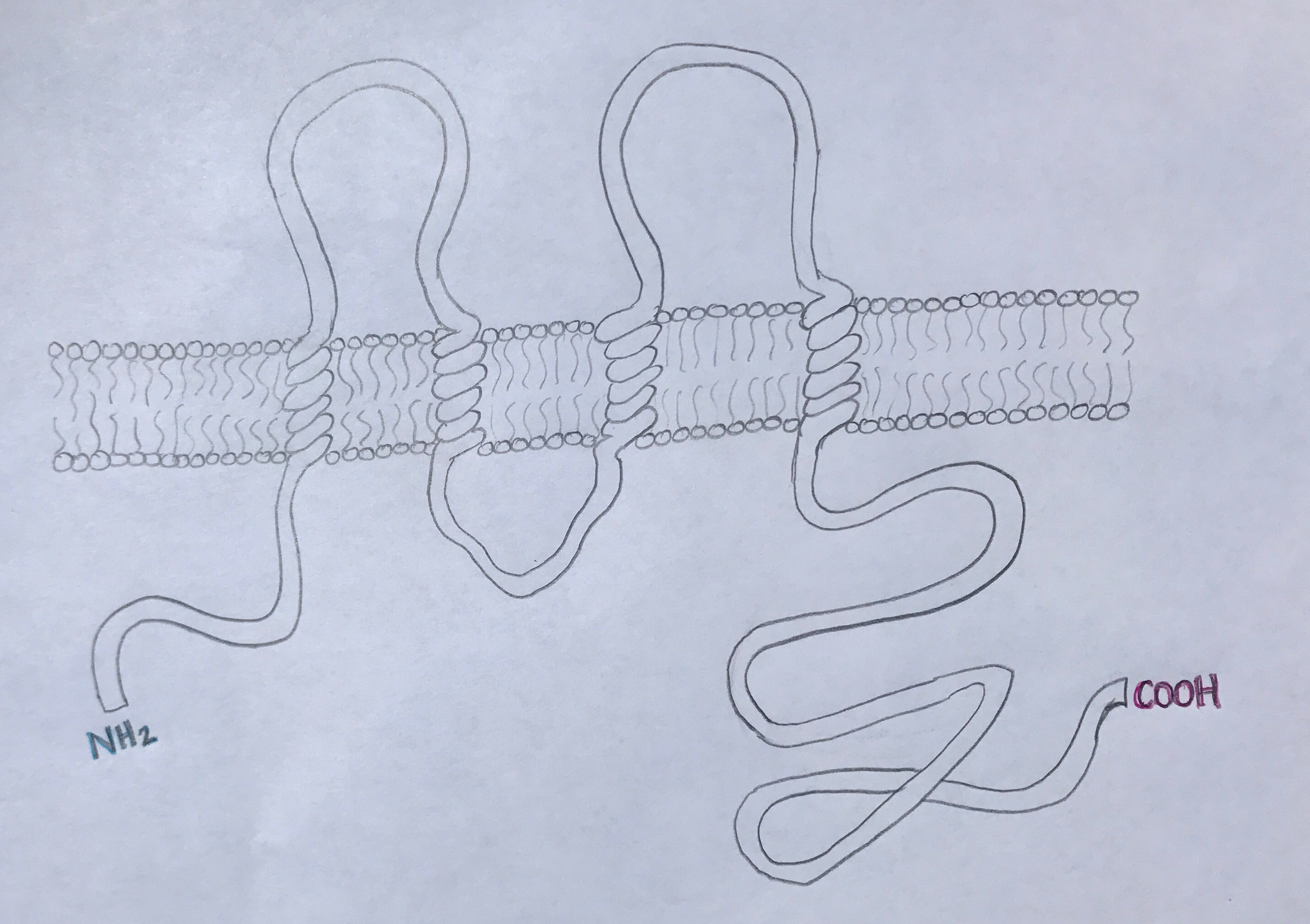
Structure
Occludin is approximately 65kDa in length. It was first identified in avian tissue, and was the first integral membrane tight junction protein to be found. Studies have found that occludin structure has 90% homology among mammals [3]. Occludin has four transmembrane alpha-helices, which are responsible for escorting occluding to tight junctions between cells [2]. As can be viewed in the image below, both the amino terminus and carboxyl terminus are located within the cytosol. The C-terminus of occluding is believed to interact with tight junction protein-1 (ZO-1), which is involved in cell-cell signaling [4].
Regulation
As mentioned before, occludin is essential in tight junctions between cells. However, it alone is not sufficient to create a tight junction. Occludin associates with two claudin proteins, which together have been found to be essential to forming a tight junction strand. Other studies found that ZO-1 and occludin together mediate cell adhesion, indicating the pivotal role occludin plays in cell-cell adhesion and tight junctions [6].
It has been found that occluding expression can be regulated by posttranscriptional methods such as gene splicing. Gene splicing also leads to an isoform of occludin that extends the amino terminus by 56 amino acids [2]. Experimental regulation of occludin has helped reveal and confirm possible functions. When the sequence of one of the extracellular loops of occludin was neutralized and disrupted, cell-cell adhesion was interfered with [6].
Occludin is also subject to posttranslational regulation. Proteolytic cleavage of occludin leads to barrier disruption, which often leads to disease and restructure of tissue framework. Occludin can be regulated by reversible phosphorylation. Phosphorylation of key serine, tyrosine, and threonine occludin residues is essential in tight junction construction [2]. Occludin phosphorylation corresponds with vessel barrier dysfunction [7].
Disease
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>