We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Gag polyprotein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
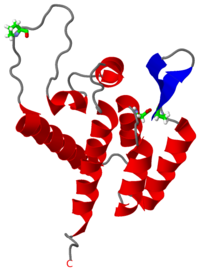
[[Image:1gwp.png|left|200px|thumb|The mature CA<sup>N</sup> domain of the [http://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(isolate_12) HIV-1] Gag<sup>283</sup> polyprotein ([[1gwp]]), is a protein involved in formation of the capsid core particle. The structure of the CA<sup>N</sup> domain was determined through [[NMR]] by Tang et al. <ref name="source">PMID:12032547</ref>.]] | [[Image:1gwp.png|left|200px|thumb|The mature CA<sup>N</sup> domain of the [http://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(isolate_12) HIV-1] Gag<sup>283</sup> polyprotein ([[1gwp]]), is a protein involved in formation of the capsid core particle. The structure of the CA<sup>N</sup> domain was determined through [[NMR]] by Tang et al. <ref name="source">PMID:12032547</ref>.]] | ||
| - | + | ||
| + | <StructureSection load='2wlv' size='450' side='right' caption='Gag polyprotein N-terminal capsid domain of HIV-2 (PDB entry [[2wlv]])' scene=''> | ||
| Line 45: | Line 46: | ||
==Implications== | ==Implications== | ||
HIV-1 viral particles need to form a capsid cone-like structure prior to infection of the host cell. The protealytic cleavage of the immature Gag<sup>283</sup> polyprotein results in a capsid domain. This post-translational modification is essential to the formation of the core structure. Many studies have shown that the β-hairpin formed after maturation is essential for the capsid core particle formation <ref name="gitti"/><ref name="von"/>. As a result of the β-hairpin formation, the helix 6 is displaced causing an allosteric mechanism for CpyA binding. Overall, the maturation of Gag<sup>283</sup> and formation of the mature CA protein is essential for core capsid particle creation and consequently final infection. | HIV-1 viral particles need to form a capsid cone-like structure prior to infection of the host cell. The protealytic cleavage of the immature Gag<sup>283</sup> polyprotein results in a capsid domain. This post-translational modification is essential to the formation of the core structure. Many studies have shown that the β-hairpin formed after maturation is essential for the capsid core particle formation <ref name="gitti"/><ref name="von"/>. As a result of the β-hairpin formation, the helix 6 is displaced causing an allosteric mechanism for CpyA binding. Overall, the maturation of Gag<sup>283</sup> and formation of the mature CA protein is essential for core capsid particle creation and consequently final infection. | ||
| - | + | </StructureSection> | |
==3D structures of Gag polyprotein== | ==3D structures of Gag polyprotein== | ||
Revision as of 10:28, 22 May 2017
| |||||||||||
Contents |
3D structures of Gag polyprotein
Updated on 22-May-2017
Additional Resources
For additional information, see: Human Immunodeficiency Virus
Reference
- ↑ 1.0 1.1 Tang C, Ndassa Y, Summers MF. Structure of the N-terminal 283-residue fragment of the immature HIV-1 Gag polyprotein. Nat Struct Biol. 2002 Jul;9(7):537-43. PMID:12032547 doi:10.1038/nsb806
- ↑ Coffin, J., S. Hughes, and H. Varmus, Retroviruses. 1997: Cold Spring Harbor Laboratory Press.
- ↑ 3.0 3.1 Gitti RK, Lee BM, Walker J, Summers MF, Yoo S, Sundquist WI. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science. 1996 Jul 12;273(5272):231-5. PMID:8662505
- ↑ 4.0 4.1 von Schwedler UK, Stemmler TL, Klishko VY, Li S, Albertine KH, Davis DR, Sundquist WI. Proteolytic refolding of the HIV-1 capsid protein amino-terminus facilitates viral core assembly. EMBO J. 1998 Mar 16;17(6):1555-68. PMID:9501077 doi:10.1093/emboj/17.6.1555
- ↑ Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996 Jun;70(6):3551-60. PMID:8648689
- ↑ Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994 Nov 24;372(6504):363-5. PMID:7969495 doi:http://dx.doi.org/10.1038/372363a0
- ↑ Ackerson B, Rey O, Canon J, Krogstad P. Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A. J Virol. 1998 Jan;72(1):303-8. PMID:9420228
Team from University of Missouri, Columbia, MO
- Students: Zheng Wang, Allison Tegge, Xin Deng
- Advisors: Jianlin Cheng, PhD, Department of Computer Science, Informatics Institute, the Life Science Center, Interdisciplinary Plant Group, University of Missouri, Columbia
- Mentor: Chun Tang, PhD, Department of Biochemistry, University of Missouri, Columbia
NMR Equipment and the Authors
Created by Allison Tegge and David Canner