3-phosphoinositide-dependent protein kinase 1
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Structural highlights == | == Structural highlights == | ||
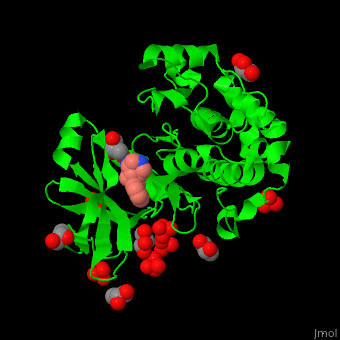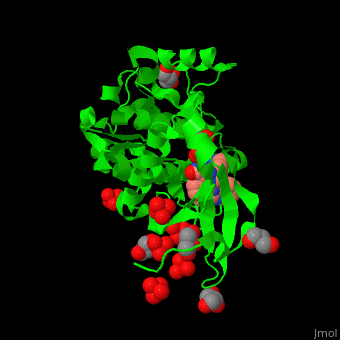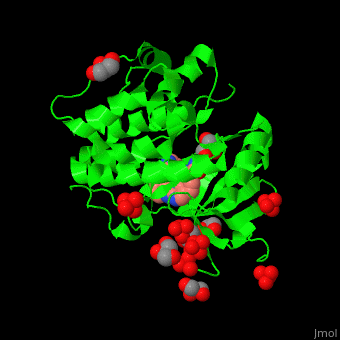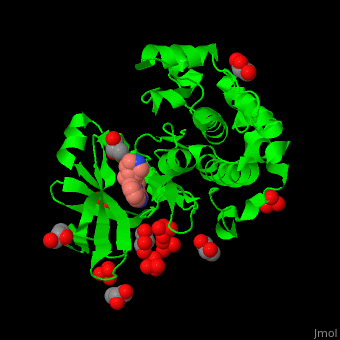
| - | Pdk1 structure contains 2 domains: kinase (residues 71-359 in human) and PH (residues 459-550) in human). The PH (Plecksin Homology) domain interacts with phospholipids. The kinase domain (kd) contains 3 binding sites. These are for substrate-binding, ATP-binding and allosteric activator docking (PIF - [Pdk1-Interacting Fragment] -pocket). | + | Pdk1 structure contains 2 domains: kinase (residues 71-359 in human) and PH (residues 459-550) in human). The PH (Plecksin Homology) domain interacts with phospholipids. The kinase domain (kd) contains 3 binding sites. These are for substrate-binding, ATP-binding and allosteric activator docking (PIF - [Pdk1-Interacting Fragment] -pocket). <scene name='54/542348/Cv/5'>Pyrazoloquinazoline inhibitor binding site</scene>. Water molecules shown as red spheres. |
</StructureSection> | </StructureSection> | ||
== 3D Structures of Pdk1== | == 3D Structures of Pdk1== | ||
Revision as of 09:42, 6 August 2017
| |||||||||||
3D Structures of Pdk1
Updated on 06-August-2017
References
- ↑ Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004 Apr;15(2):161-70. PMID:15209375