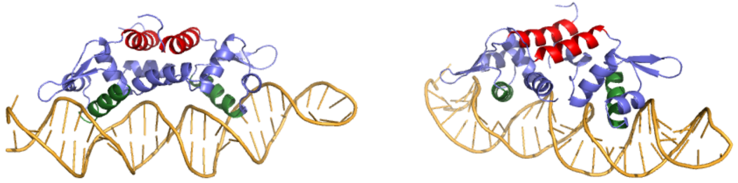Sandbox Reserved 1053
From Proteopedia
| Line 9: | Line 9: | ||
==Czr Operon== | ==Czr Operon== | ||
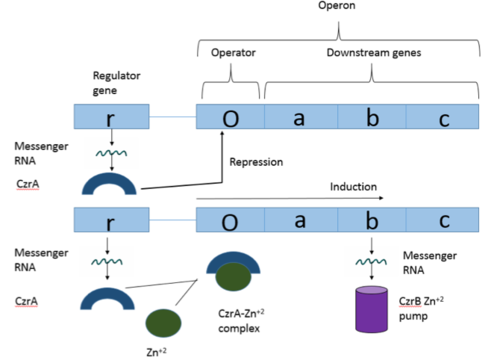
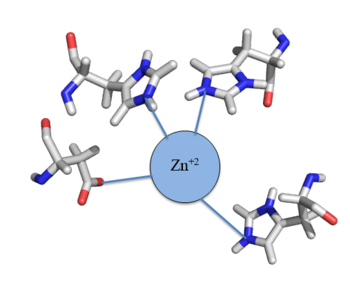
| - | The Chromosome Determined Zinc Responsible (Czr) operon acts as described above, with Czr A acting as a regulator protein to the downstream gene Czr B. The Czr B gene codes for a Zn <sup>2+</sup> pump, so Czr A is responsible for controlling the transport of Zn <sup>2+</sup> out of the cell. The role of Czr A in the Czr operon is described in further detail as part of the explanation of biological function. In addition to being a component of an operon, Czr A is also considered to be a metal sensor protein. While the immediate function of Czr A is gene regulation, this serves the larger purpose of acting to maintain an appropriate concentration of Zn <sup>2+</sup> | + | The Chromosome Determined Zinc Responsible (Czr) operon acts as described above, with Czr A acting as a regulator protein to the downstream gene Czr B. The Czr B gene codes for a Zn <sup>2+</sup> pump, so Czr A is responsible for controlling the transport of Zn <sup>2+</sup> out of the cell. The role of Czr A in the Czr operon is described in further detail as part of the explanation of biological function. In addition to being a component of an operon, Czr A is also considered to be a metal sensor protein. While the immediate function of Czr A is gene regulation, this serves the larger purpose of acting to maintain an appropriate concentration of Zn <sup>2+</sup> inside the cell membrane. |
== Biological Function == | == Biological Function == | ||
Revision as of 23:51, 18 August 2017
Czr A: A Zinc Dependent Transcriptional Regulator
Background
Operon Overview
Operons are a critical genetic component of most prokaryotic cells. There are many different operons, responsible for the production of proteins with a wide range of functions. The most well-known and studied operons are the Lac and Trp operons, responsible for producing enzymes which metabolize lactose and tryptophan respectively. Despite many differences in each operon and the proteins that they encode, operons all function in the same general manner (Figure 1). Structurally, each operon contains a regulator, an operator, and one or more structural genes. The regulator gene codes for a protein responsible for managing the expression level of the structural genes. The operator contains the binding sequence for RNA polymerase and is the site where transcription begins. Lastly, the structural genes code for proteins to be used elsewhere. The regulator protein (produced as a result of expression of the regulator gene) most often acts in a repressive manner, though this is not always the case. That is, the regulator protein will bind to the operator, inhibiting the binding and/or progression of RNA polymerase to the structural genes, thus inhibiting transcription of the genes into mRNA. If the regulator protein were always active, the structural genes would never be expressed, so there must be a way to inactive the regulator protein, thus enabling expression of the structural genes. This is usually achieved through the binding of an inhibitor to the regulator protein. Since regulator proteins are DNA binding proteins, often this inhibition is allosteric rather than competitive. The inhibitor of the regulator protein binds to somewhere other than the active site of the protein, changing the regulator protein to decreases its affinity or ability to bind DNA and repress transcription.
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 18177-18182.
- ↑ MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006 Sep;70(3):583-604. PMID:16959962 doi:http://dx.doi.org/10.1128/MMBR.00015-06
- ↑ Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun 4;4(6):1609-1614.
- ↑ Grossoehme NE, Giedroc DP. Energetics of allosteric negative coupling in the zinc sensor S. aureus CzrA. J Am Chem Soc. 2009 Dec 16;131(49):17860-70. doi: 10.1021/ja906131b. PMID:19995076 doi:http://dx.doi.org/10.1021/ja906131b