Odorant binding protein
From Proteopedia
| Line 30: | Line 30: | ||
The main purpose in the adult moth's short life is reproduction. In fact, the male and female moth invest all of their energy and resources hoping to reach to the ultimate goal- mating. This long journey begins when the female moth releases a sex pheromone, usually during specific hours in the night <ref>doi: 10.1007/BF01946910</ref>. | The main purpose in the adult moth's short life is reproduction. In fact, the male and female moth invest all of their energy and resources hoping to reach to the ultimate goal- mating. This long journey begins when the female moth releases a sex pheromone, usually during specific hours in the night <ref>doi: 10.1007/BF01946910</ref>. | ||
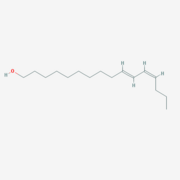
BmorPBP was first identified in the ''B. mori'' male antennae by Krieger et al. in 1996 <ref>doi: 10.1016/0965-1748(95)00096-8</ref>, as the PBP of the first sex pheromone discovered ((E,Z)-10,12-hexadecadienol, or [http://en.wikipedia.org/wiki/Bombykol Bombykol]). The male moth needs to detect minute amount of the pheromone in the air, while following a turbulent wind-born pheromone trail and response fast (experimental evidence shows a response time of 0.5 seconds<ref>doi: 10.1038/293161a0</ref>). | BmorPBP was first identified in the ''B. mori'' male antennae by Krieger et al. in 1996 <ref>doi: 10.1016/0965-1748(95)00096-8</ref>, as the PBP of the first sex pheromone discovered ((E,Z)-10,12-hexadecadienol, or [http://en.wikipedia.org/wiki/Bombykol Bombykol]). The male moth needs to detect minute amount of the pheromone in the air, while following a turbulent wind-born pheromone trail and response fast (experimental evidence shows a response time of 0.5 seconds<ref>doi: 10.1038/293161a0</ref>). | ||
| - | + | ||
==BmorPBP structure and function== | ==BmorPBP structure and function== | ||
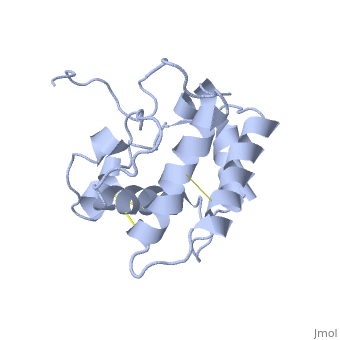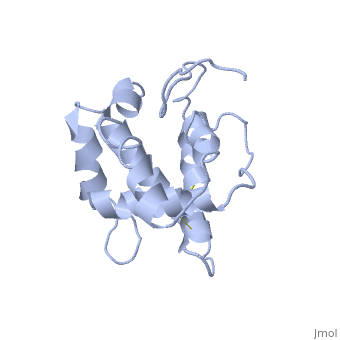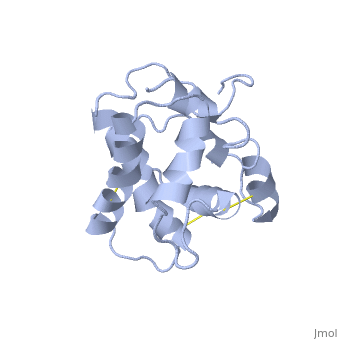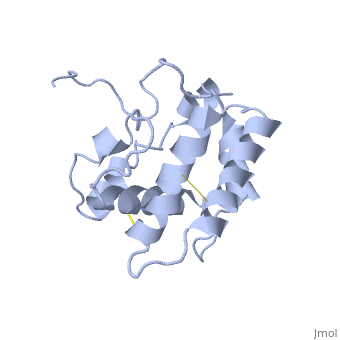
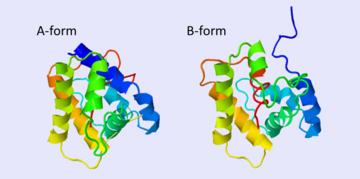
The protein has 164 amino acids that forms 6-7 alpha helices (depends on the protein conformation). Three <font color=#FFEF00><b>disulfide bonds</b></font> formed by <scene name='68/683383/Cysteins6/1'>6 cystein </scene> residues tied four helices, and form the compact and robust structure of the protein. As expected from a soluble protein, its surface is covered with <scene name='68/683383/Charged_resid/1'>charged residues</scene>, which allows it to interact with the water molecule and solubilize in the sensillar lymph. | The protein has 164 amino acids that forms 6-7 alpha helices (depends on the protein conformation). Three <font color=#FFEF00><b>disulfide bonds</b></font> formed by <scene name='68/683383/Cysteins6/1'>6 cystein </scene> residues tied four helices, and form the compact and robust structure of the protein. As expected from a soluble protein, its surface is covered with <scene name='68/683383/Charged_resid/1'>charged residues</scene>, which allows it to interact with the water molecule and solubilize in the sensillar lymph. | ||
| Line 62: | Line 62: | ||
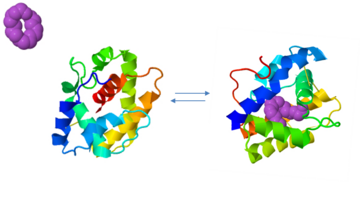
==Activation by the complex pheromone-PBP== | ==Activation by the complex pheromone-PBP== | ||
An alternative mode of action was proposed for the receptor activation in ''Drosophila melanogaster'', where it was found that the complex of pheromone-PBP is required for the activity of pheromone-sensitive neurons <ref>DOI: 10.1016/j.neuron.2004.12.031</ref><ref>doi: 10.1016/j.cell.2008.04.046</ref> | An alternative mode of action was proposed for the receptor activation in ''Drosophila melanogaster'', where it was found that the complex of pheromone-PBP is required for the activity of pheromone-sensitive neurons <ref>DOI: 10.1016/j.neuron.2004.12.031</ref><ref>doi: 10.1016/j.cell.2008.04.046</ref> | ||
| - | + | </StructureSection> | |
==3D structure of odorant binding protein== | ==3D structure of odorant binding protein== | ||
Revision as of 12:04, 23 August 2017

Contents |
Introduction
Odorant-binding protein (OBP) are soluble proteins which are involved in the processes of odorant detection in the olfactory sensilla [1]. Though functionally the same, vertebrates and insects OBP have different origin and structure. OBPs are important for insect olfaction. For instance, OBP76a (LUSH) in the fly Drosophila melanogaster is required for the detection of the pheromone vaccenyl acetate [2] and has been proven to adopt a conformation that activates the odorant receptor [3].
OBP Function
Despite five decades of intensive research, the exact roles of OBP and the mechanism by which the odorant receptor (OR) is activated are still in dispute [4][5].
A few functions have been suggested for OBP:
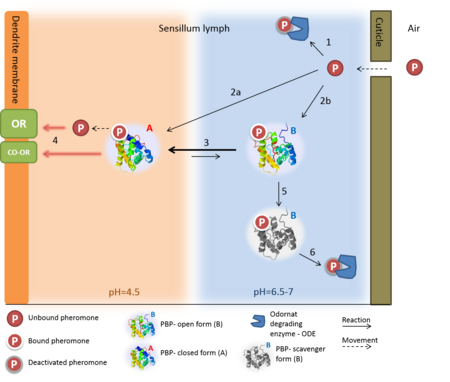
1. Solubilizing the odorant molecule and its transportation in the sensillar lymph.
2. Protecting the odorant molecule from the odorant degrading enzymes, in the sensillar lymph.
3. Activating the odorant receptor on the dendrite membrane, by the odorant-OBP complex.
4. Mediating the deactivation of the odorant molecule after the activation of the receptor.
5. An organic anion (the protein has 9 negative charges).
Of all, the first role of OBP as an odorant solubilizer and carrier is generally accepted.
In order to explain the structure and function of these fascinating proteins, this page will further focus on a particular OBP - the well investigated Bombyx mori pheromone binding protein: BmorPBP.
Bombyx mori BmorPBP (lets talk about sex..)
| |||||||||||
3D structure of odorant binding protein
Updated on 23-August-2017
See also
- Odorant_binding_protein_3D_structures
- Chemical communication in arthropods
- Pheromone binding protein
- Bovine odorant binding protein
References
Proteopedia Page Contributors and Editors (what is this?)
Nurit Eliash, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky