We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Phosphate-binding protein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' scene='48/488458/Cv/1' caption='E. coli phosphate-binding protein complex with phosphate [[1pbp]]'> |
== Function == | == Function == | ||
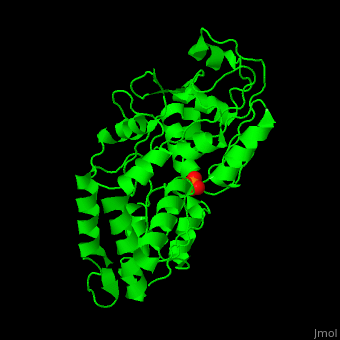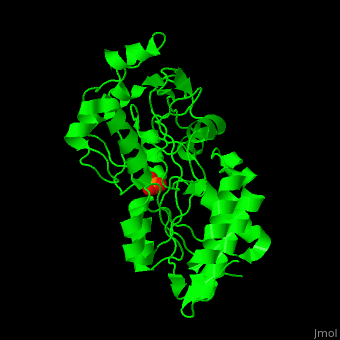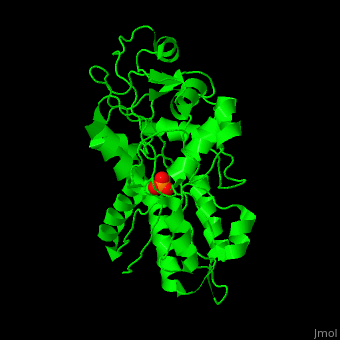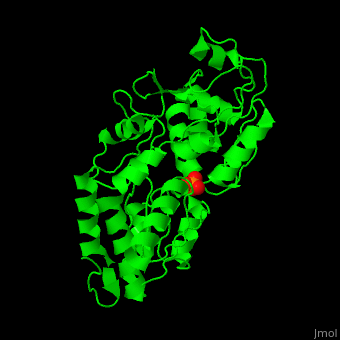
'''Phosphate-binding protein''' (PBP) binds phosphate (Pi) with high affinity. PBP is involved in various biological processes like cell cycle regulation. PBP is produced under conditions of low Pi concentration. It binds Pi in the periplasm and transfers it to a membrane protein which transports it to the cytoplasm. PBP undergoes conformational change upon binding to Pi<ref>PMID:25338617</ref>. | '''Phosphate-binding protein''' (PBP) binds phosphate (Pi) with high affinity. PBP is involved in various biological processes like cell cycle regulation. PBP is produced under conditions of low Pi concentration. It binds Pi in the periplasm and transfers it to a membrane protein which transports it to the cytoplasm. PBP undergoes conformational change upon binding to Pi<ref>PMID:25338617</ref>. | ||
Revision as of 15:05, 31 August 2017
| |||||||||||
3D structures of phosphate-binding protein
Updated on 31-August-2017
1qui, 1oib - EcPBP (mutant) - Escherichia coli
4jwo – PBP – Planctomyces limnophilus
4exl, 4h1x – SpPBP – Streptococcus pneumoniae
1pbp, 2abh, 1ixh – EcPBP + Pi
1qui - EcPBP (mutant) + Br + Pi
1quj, 1qul - EcPBP (mutant) + Cl + Pi
1quk, 1ixi, 1ixg, 1a40 - EcPBP (mutant) + Pi
1a54, 1a55 - EcPBP (mutant) + dihydrogenphosphate
2v3q – hPBP + Pi – human
1pc3, 4lvq – PBP + Pi – Mycobacterium tuberculosis
3w9v, 3w9w – upPBP + Pi – unidentified prokaryote
4lat – SpPBP + Pi
4m1v - upPBP (mutant) + Pi
4omb, 4pqj – PBP + Pi – Pseudomonas aeruginosa
5wnn – PBP + Pi – Brucella melitensis
5i84 – PBP + Pi – Xanthomonas citri
References
- ↑ Gonzalez D, Richez M, Bergonzi C, Chabriere E, Elias M. Crystal structure of the phosphate-binding protein (PBP-1) of an ABC-type phosphate transporter from Clostridium perfringens. Sci Rep. 2014 Oct 16;4:6636. doi: 10.1038/srep06636. PMID:25338617 doi:http://dx.doi.org/10.1038/srep06636
- ↑ Wang Z, Choudhary A, Ledvina PS, Quiocho FA. Fine tuning the specificity of the periplasmic phosphate transport receptor. Site-directed mutagenesis, ligand binding, and crystallographic studies. J Biol Chem. 1994 Oct 7;269(40):25091-4. PMID:7929197