This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1wki
From Proteopedia
| Line 7: | Line 7: | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= RPLP ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=274 Thermus thermophilus]) | |GENE= RPLP ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=274 Thermus thermophilus]) | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1wki FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1wki OCA], [http://www.ebi.ac.uk/pdbsum/1wki PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1wki RCSB]</span> | ||
}} | }} | ||
| Line 37: | Line 40: | ||
[[Category: structural genomic]] | [[Category: structural genomic]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Mon Mar 31 00:37:32 2008'' |
Revision as of 21:37, 30 March 2008
| |||||||
| Gene: | RPLP (Thermus thermophilus) | ||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
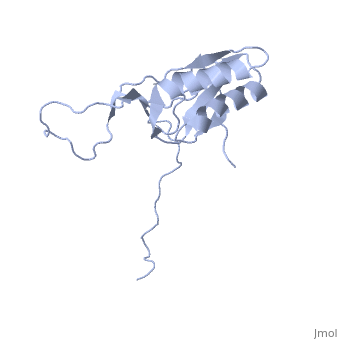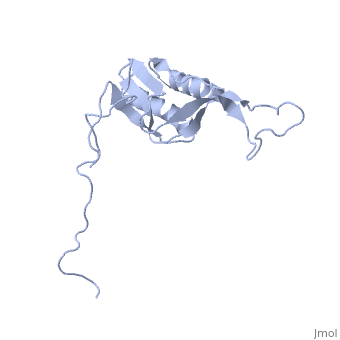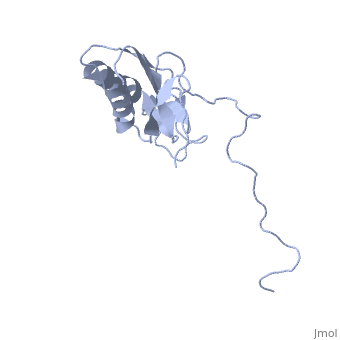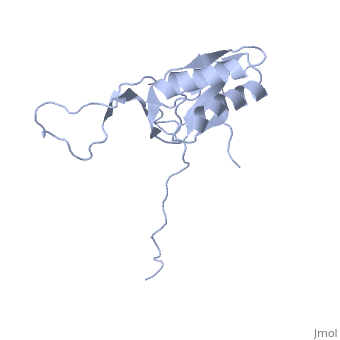
solution structure of ribosomal protein L16 from thermus thermophilus HB8
Overview
Ribosomal protein L16 is an essential component of the bacterial ribosome. It organizes the architecture of aminoacyl tRNA binding site in the ribosome 50S subunit. The three-dimensional structure of L16 from Thermus thermophilus HB8 was determined by NMR. In solution, L16 forms an alpha+beta sandwich structure combined with two additional beta sheets located at the loop regions connecting the two layers. The terminal regions and a central loop region did not show any specific secondary structure. The structured part of L16 could be superimposed well on the C(alpha) model of L16 determined in the crystal structure of the ribosome 50S subunit. By overlaying the L16 solution structure onto the coordinates of the ribosome crystal structure, we constructed the combined model that represents the ribosome-bound state of L16 in the detailed structure. The model showed that L16 possesses residues in contact with helices 38, 39, 42, 43 and 89 of 23S rRNA and helix 4 of 5S rRNA. This suggests its broad effect on the ribosome architecture. Comparison of L16 with the L10e protein, which is the archaeal counterpart, showed that they share a common fold, but differ in some regions of functional importance, especially in the N-terminal region. All known mutation sites in L16 that confer resistance to avilamycin and evernimicin were positioned so that their side-chains were exposed to solvent in the internal cavity of the ribosome. This suggests the direct participation of L16 as a part of the binding site for antibiotics.
About this Structure
1WKI is a Protein complex structure of sequences from Thermus thermophilus. Full crystallographic information is available from OCA.
Reference
Solution structure of ribosomal protein L16 from Thermus thermophilus HB8., Nishimura M, Yoshida T, Shirouzu M, Terada T, Kuramitsu S, Yokoyama S, Ohkubo T, Kobayashi Y, J Mol Biol. 2004 Dec 10;344(5):1369-83. PMID:15561149
Page seeded by OCA on Mon Mar 31 00:37:32 2008
Categories: Protein complex | Thermus thermophilus | Kobayashi, Y. | Kuramitsu, S. | Nishimura, M. | Ohkubo, T. | RSGI, RIKEN Structural Genomics/Proteomics Initiative. | Shirouzu, M. | Terada, T. | Yokoyama, S. | Yoshida, T. | Mixed alpha/beta | Ribosome | Riken structural genomics/proteomics initiative | Rsgi | Structural genomic