We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Retroviral Integrase
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
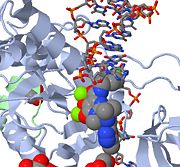
[[Image:Strand Inhibition.jpg|thumb|alt=Alt text|Above is a JMOL image of MK-0518 blocking the 3' end of the viral DNA from binding the active site. Notice the metal chelating oxygen atoms in MK-0518 interacting with the magnesium cations found in the active site.]] | [[Image:Strand Inhibition.jpg|thumb|alt=Alt text|Above is a JMOL image of MK-0518 blocking the 3' end of the viral DNA from binding the active site. Notice the metal chelating oxygen atoms in MK-0518 interacting with the magnesium cations found in the active site.]] | ||
| - | HIV integrase type 1 is a relatively new and novel target for inhibitors. In 2007, the first HIV-1 integrase inhibitor, Raltegravir, was approved by the FDA for use in HIV-1 as a combination therapy <ref>[http://www.prn.org/index.php/management/article/integrase_inhibitors_raltegravir_elvitegravir_hiv_disease_478 | + | HIV integrase type 1 is a relatively new and novel target for inhibitors. In 2007, the first HIV-1 integrase inhibitor, Raltegravir, was approved by the FDA for use in HIV-1 as a combination therapy <ref>James F. Braun, DO, Ruth J. Cronje, PhD, Marnie G. Henderson [http://www.prn.org/index.php/management/article/integrase_inhibitors_raltegravir_elvitegravir_hiv_disease_478 HIV-1 Integrase Inhibitors Inhibitors (2008)]. www.prn.org Volume 13, Pages 1–9</ref>. Strand-transfer inhibitors work by preventing the concerted integration of the viral DNA into the host chromosome. After viral entry into the host cell, the reverse transcriptase converts the viral ssRNA into dsDNA. At this point, the integrase forms a complex with the viral DNA, creating the pre-integration complex (intasome). The pre-integration complex is then chaperoned into the nucleus, where two nucleotides are excised from the 3' end. Next, the DNA is then covalently integrated into the host DNA. The strand-transfer inhibitors interrupt this process, preventing the integration of the viral DNA into the host chromosome. Strand-transfer inhibitors work by engaging the metal ion cofactors found in the retroviral integrase active site. The metal chelating oxygen atoms found in the inhibitors interact directly with the metal cofactors, while the halobenzyl group fits into the pocket created by the displaced 3' viral DNA in the active site. |
==HIV and AIDS== | ==HIV and AIDS== | ||
Revision as of 18:23, 27 September 2017
| |||||||||||
Integrase Inhibitors
| Name | Brand | Company | Patent | Notes |
| Raltegravir | Isentress | Merck & Co. | - | also known as MK-0518. The isopropyl and methyl-oxadiazole of MK-0518 are involved in hydrophobic and stacking interactions with side chains of Pro 214 and Tyr 212 to stabilize this drug within the PFV intasome active site. This manner of drug-binding interaction causes displacement of the reactive 3' viral DNA end from the active site of PFV intasome. After binding of MK-0518 to active site, the reactive 3' hydroxyl group moves away from the active site of the PFV intasome by more than 6 Angstroms. Raltegravir was approved by the FDA on October 12, 2007, for use with other anti-HIV agents in the treatment of HIV infection in adults. It is the first integrase inhibitor approved by the FDA. |
| Elvitegravir | - | Gilead Science | - | GS-9137 interacts with Pro 214 of PFV intasome through its quinolone base and isopropyl group. In experimental stages; shares the core structure of quinolone antibiotics. Phase II studies of elvitegravir in people who are treatment experienced have been completed. Phase III studies in treatment experienced patients are ongoing. A phase II study of elvitegravir in people who have never taken antiretroviral therapy is underway. This study will also be evaluated a boosting agent in place of Norvir, currently called GS9350. Elvitegravir holds promise for HIV-positive patients who have taken other anti-HIV drugs in the past. |
| MK-2048 | - | Merck & Co. | - | A second generation integrase inhibitor, intended to be used against HIV infection. It is superior to the first available integrase inhibitor, raltegravir, in that it inhibits the HIV enzyme integrase 4 times longer. It is being investigated for use as part of pre-exposure prophylaxis (PrEP). |
See also Retroviral Integrase Inhibitor Pharmacokinetics[6].
Additional Resources
For additional information, see: Human Immunodeficiency Virus
3D Structures of Retroviral Integrase
Updated on 27-September-2017
References
- ↑ Pandey KK, Grandgenett DP. HIV-1 Integrase Strand Transfer Inhibitors: Novel Insights into their Mechanism of Action. Retrovirology (Auckl). 2008 Nov 5;2:11-16. PMID:19915684
- ↑ 2.0 2.1 2.2 2.3 Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature. 2010 Mar 11;464(7286):232-6. Epub 2010 Jan 31. PMID:20118915 doi:10.1038/nature08784
- ↑ deJesus, Edwin HIV Antiretroviral Agents in Development. The Body: The Complete HIV/AIDS Resource. March 30, 2006
- ↑ James F. Braun, DO, Ruth J. Cronje, PhD, Marnie G. Henderson HIV-1 Integrase Inhibitors Inhibitors (2008). www.prn.org Volume 13, Pages 1–9
- ↑ AIDS-Info
- ↑ Iwamoto M, Wenning LA, Petry AS, Laethem M, De Smet M, Kost JT, Breidinger SA, Mangin EC, Azrolan N, Greenberg HE, Haazen W, Stone JA, Gottesdiener KM, Wagner JA. Minimal effects of ritonavir and efavirenz on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2008 Dec;52(12):4338-43. Epub 2008 Oct 6. PMID:18838589 doi:10.1128/AAC.01543-07
3. deJesus, Edwin HIV Antiretroviral Agents in Development. The Body: The Complete HIV/AIDS Resource. March 30, 2006.
4. AIDS Info
Further Reading
- GEN News Highlights "Scientists Solve 3-D Crystal Structure of Retroviral Integrase Bound to Viral DNA", Genetic Engineering & Biotechnology News February 1, 2010.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Rhysly Martinez, Joel L. Sussman, Alexander Berchansky, David Canner, Jordan Heard, Eugene Babcock, Garrett Asanuma