Ribosomal protein L3
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
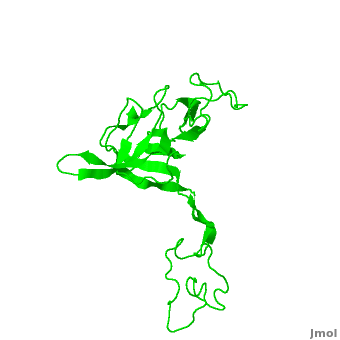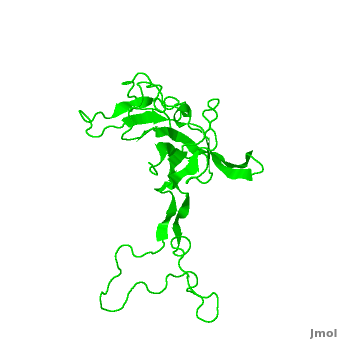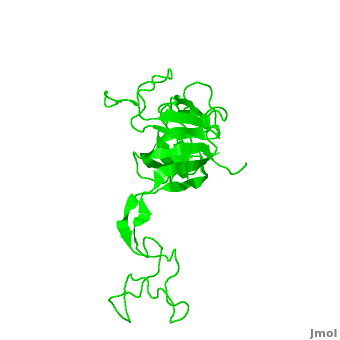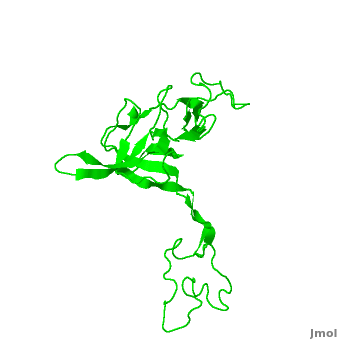
<StructureSection load='2ogm' size='350' side='right' scene='Ribosomal_protein_L3/Cv/1' caption='Ribosomal protein L3 complex with 23S RNA and antibacterial drug (PDB code [[2ogm]])'> | <StructureSection load='2ogm' size='350' side='right' scene='Ribosomal_protein_L3/Cv/1' caption='Ribosomal protein L3 complex with 23S RNA and antibacterial drug (PDB code [[2ogm]])'> | ||
== Function == | == Function == | ||
| - | [[Ribosomal protein L3]] (RPL3 ) is one of the 34 proteins which belong to the <scene name=' | + | [[Ribosomal protein L3]] (RPL3 ) is one of the 34 proteins which belong to the <scene name='42/425998/Cv/4'>large 50S subunit</scene> of the prokaryotic ribosome or to the ca. 49 proteins in the large 60S subunit of the eukaryotic ribosome. It binds to 23S RRNA near the 3'-end where it nucleates the assembly of the large subunit<ref>PMID:17194937</ref>. In the archaea like in ''Haloarcula marismortui'' the L3 is called L3P (RPL3P). |
== Relevance == | == Relevance == | ||
Revision as of 12:22, 1 October 2017
| |||||||||||
3D Structures of Ribosomal protein L3
Updated on 01-October-2017
2ogm, 2ogn, 2ogo – RPL3 + 23S RRNA + antibiotic – Deinococcus radiodurans
References
- ↑ Petrov A, Meskauskas A, Dinman JD. Ribosomal protein L3: influence on ribosome structure and function. RNA Biol. 2004 May;1(1):59-65. Epub 2004 May 1. PMID:17194937
- ↑ Pringle M, Poehlsgaard J, Vester B, Long KS. Mutations in ribosomal protein L3 and 23S ribosomal RNA at the peptidyl transferase centre are associated with reduced susceptibility to tiamulin in Brachyspira spp. isolates. Mol Microbiol. 2004 Dec;54(5):1295-306. PMID:15554969 doi:http://dx.doi.org/10.1111/j.1365-2958.2004.04373.x