This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Transketolase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
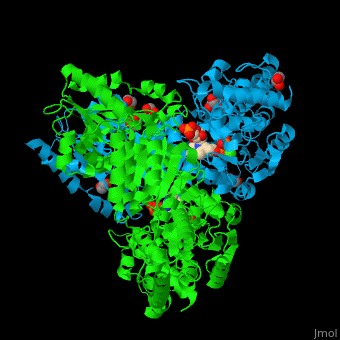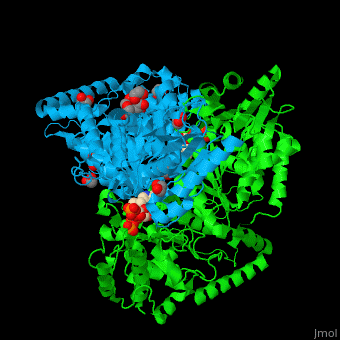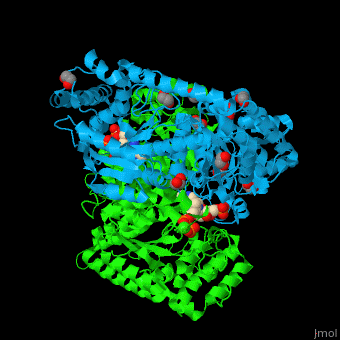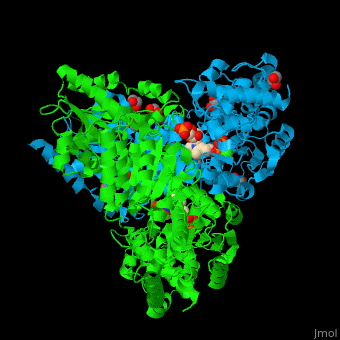
| - | <StructureSection load=' | + | <StructureSection load='' size='450' side='right' caption='E. coli transketolase 1 dimer complex with xylulose-5-phosphate-thiamine diphosphate adduct, ethylene glycol and Ca+2 ion (green) (PDB code [[2r8o]]).' scene='46/466534/Cv/1'> |
== Function == | == Function == | ||
'''Transketolase''' (TKT) catalyzes two opposite reactions. The synthesis of sedoheptulose-7-P in the pentose phosphate pathway using thiamine diphosphate (TPP) as co-factor; and the conversion of sedoheptulose-7-P and glyceraldehyde-3-P to aldose and ketose in the Calvin cycle<ref>PMID:8369276</ref>. | '''Transketolase''' (TKT) catalyzes two opposite reactions. The synthesis of sedoheptulose-7-P in the pentose phosphate pathway using thiamine diphosphate (TPP) as co-factor; and the conversion of sedoheptulose-7-P and glyceraldehyde-3-P to aldose and ketose in the Calvin cycle<ref>PMID:8369276</ref>. | ||
Revision as of 05:01, 16 October 2017
| |||||||||||
3D Structures of transketolase
Updated on 16-October-2017
References
- ↑ Verschueren KH, Kingma J, Rozeboom HJ, Kalk KH, Janssen DB, Dijkstra BW. Crystallographic and fluorescence studies of the interaction of haloalkane dehalogenase with halide ions. Studies with halide compounds reveal a halide binding site in the active site. Biochemistry. 1993 Sep 7;32(35):9031-7. PMID:8369276
- ↑ Asztalos P, Parthier C, Golbik R, Kleinschmidt M, Hubner G, Weiss MS, Friedemann R, Wille G, Tittmann K. Strain and near attack conformers in enzymic thiamin catalysis: X-ray crystallographic snapshots of bacterial transketolase in covalent complex with donor ketoses xylulose 5-phosphate and fructose 6-phosphate, and in noncovalent complex with acceptor aldose ribose 5-phosphate. Biochemistry. 2007 Oct 30;46(43):12037-52. Epub 2007 Oct 3. PMID:17914867 doi:10.1021/bi700844m