We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Transferrin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
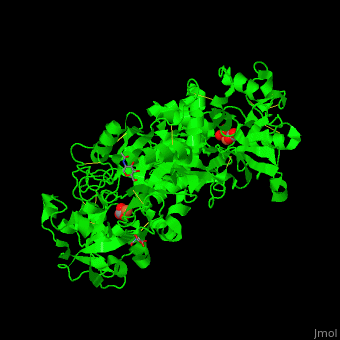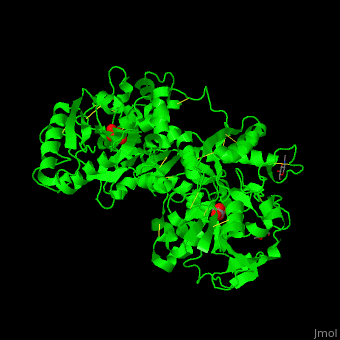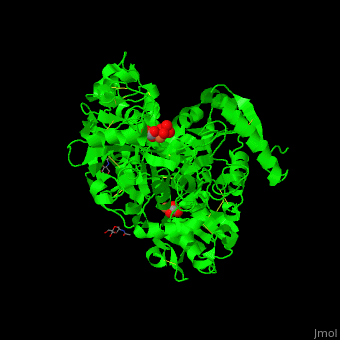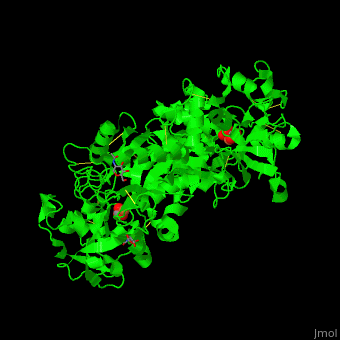
| - | <StructureSection load=' | + | <StructureSection load='' size='400' side='right' caption='Glycosylated human transferrin with Fe+3 (light red), carbonate and sulfate (PDB code [[3qyt]]).' scene='48/480879/Cv/1' pspeed='8'> |
== Function == | == Function == | ||
'''Transferrin''' or '''serotransferrin''' (TF) is an iron-binding protein. TF delivers iron from absorption centers in the duodendum and white blood cell macrophages to all tissues<ref>PMID:2208733</ref>. '''Ovotransferrin''' (OTF) is a glycosylated TF of egg white. For details see [[Molecular Playground/Transferrin]]. | '''Transferrin''' or '''serotransferrin''' (TF) is an iron-binding protein. TF delivers iron from absorption centers in the duodendum and white blood cell macrophages to all tissues<ref>PMID:2208733</ref>. '''Ovotransferrin''' (OTF) is a glycosylated TF of egg white. For details see [[Molecular Playground/Transferrin]]. | ||
Revision as of 05:06, 16 October 2017
| |||||||||||
3D Structures of Transferrin
Updated on 16-October-2017
References
- ↑ de Jong G, van Dijk JP, van Eijk HG. The biology of transferrin. Clin Chim Acta. 1990 Sep;190(1-2):1-46. PMID:2208733
- ↑ Liu YS, Xu GY, Cheng DQ, Li YM. Determination of serum carbohydrate-deficient transferrin in the diagnosis of alcoholic liver disease. Hepatobiliary Pancreat Dis Int. 2005 May;4(2):265-8. PMID:15908327
- ↑ Yang N, Zhang H, Wang M, Hao Q, Sun H. Iron and bismuth bound human serum transferrin reveals a partially-opened conformation in the N-lobe. Sci Rep. 2012;2:999. doi: 10.1038/srep00999. Epub 2012 Dec 19. PMID:23256035 doi:10.1038/srep00999