2-Oxoglutarate Dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='2jgd' size='400' side='right' scene='' caption='Dimer of E. coli 2-oxogluterate dehydrogenase E1 component complex with AMP | + | <StructureSection load='2jgd' size='400' side='right' scene='' caption='Dimer of E. coli 2-oxogluterate dehydrogenase E1 component complex with AMP [[2jgd]]'> |
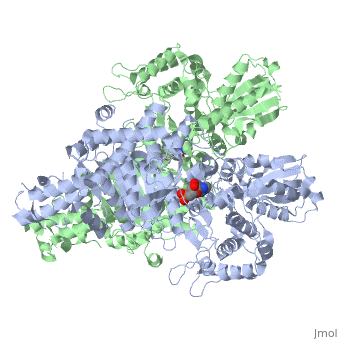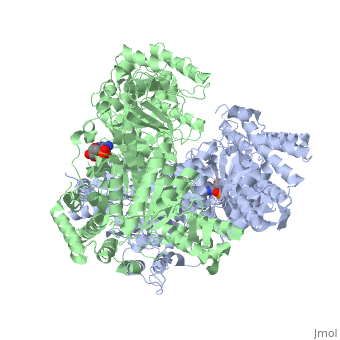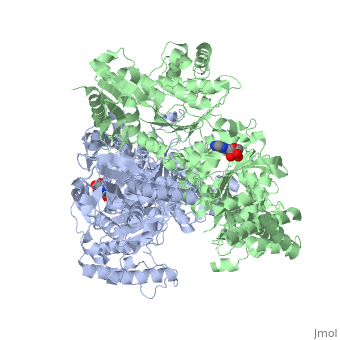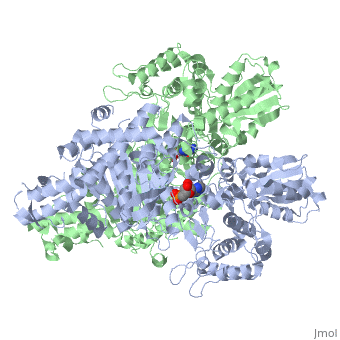
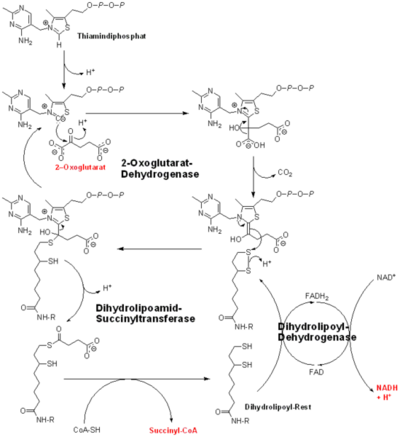
'''2-oxoglutarate dehydrogenase''' is a complex of three components: E1, E2 and E3. The enzyme '''2-Oxoglutarate Dehydrogenase E1o''' (OGDH) is a subunit the 2-Oxoglutarate Dehydrogenase ([[EC Number]] [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.2.4.2 1.2.4.2]) multi enzyme complex. This subunit is a homo-dimer and one of three enzymes that make up the multi-enzyme complex of 2-Oxoglutarate Dehydrogenase. Each of the <scene name='Lucas_Evans_Sandbox/Monomer/2'>monomers</scene> that make up the homo-dimer have an <scene name='Lucas_Evans_Sandbox/Amp1/1'>adenosine monophosphate</scene> cofactor that facilitates the catalysis. E1o is not categorized in the Structural Classification of Proteins (SCOP); however, the <scene name='Lucas_Evans_Sandbox/Secondary_structure/2'>secondary structure</scene> of one of the dimers shows that this enzyme has large sections of alpha-helices followed by a large sections of parallel beta-pleated sheets these alpha and beta subunits are fused as a single polypeptide <ref name="one">PMID:17367808 </ref>. E2 component is '''dihydrolipoyl succinyltransferase''' or '''dihydrolipoamide succinyltransferase''' (DLST) with lipoic acid as coenzyme and E3 is [[Dihydrolipoamide dehydrogenase]] (DLD) with FAD as coenzyme. The 2-oxoglutaqrate dehydrogenase complex participates in then citric acid cycle, lysine degradation and tryptophan metabolism. See also [[Krebs cycle step 4]]. | '''2-oxoglutarate dehydrogenase''' is a complex of three components: E1, E2 and E3. The enzyme '''2-Oxoglutarate Dehydrogenase E1o''' (OGDH) is a subunit the 2-Oxoglutarate Dehydrogenase ([[EC Number]] [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.2.4.2 1.2.4.2]) multi enzyme complex. This subunit is a homo-dimer and one of three enzymes that make up the multi-enzyme complex of 2-Oxoglutarate Dehydrogenase. Each of the <scene name='Lucas_Evans_Sandbox/Monomer/2'>monomers</scene> that make up the homo-dimer have an <scene name='Lucas_Evans_Sandbox/Amp1/1'>adenosine monophosphate</scene> cofactor that facilitates the catalysis. E1o is not categorized in the Structural Classification of Proteins (SCOP); however, the <scene name='Lucas_Evans_Sandbox/Secondary_structure/2'>secondary structure</scene> of one of the dimers shows that this enzyme has large sections of alpha-helices followed by a large sections of parallel beta-pleated sheets these alpha and beta subunits are fused as a single polypeptide <ref name="one">PMID:17367808 </ref>. E2 component is '''dihydrolipoyl succinyltransferase''' or '''dihydrolipoamide succinyltransferase''' (DLST) with lipoic acid as coenzyme and E3 is [[Dihydrolipoamide dehydrogenase]] (DLD) with FAD as coenzyme. The 2-oxoglutaqrate dehydrogenase complex participates in then citric acid cycle, lysine degradation and tryptophan metabolism. See also [[Krebs cycle step 4]]. | ||
Revision as of 16:41, 18 October 2017
| |||||||||||
3D structures of 2-Oxoglutarate dehydrogenase
Updated on 18-October-2017
References
- ↑ 1.0 1.1 1.2 1.3 Frank RA, Price AJ, Northrop FD, Perham RN, Luisi BF. Crystal structure of the E1 component of the Escherichia coli 2-oxoglutarate dehydrogenase multienzyme complex. J Mol Biol. 2007 May 4;368(3):639-51. Epub 2007 Feb 7. PMID:17367808 doi:10.1016/j.jmb.2007.01.080
- ↑ 2.0 2.1 McMinn CL, Ottaway JH. Studies on the mechanism and kinetics of the 2-oxoglutarate dehydrogenase system from pig heart. Biochem J. 1977 Mar 1;161(3):569-81. PMID:192200
- ↑ Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, Miyakawa H, Norman GL, Lee W, Gershwin ME. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007 Nov;46(5):1436-42. PMID:17657817 doi:10.1002/hep.21828
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. p.580
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Lucas Evans, Alexander Berchansky, Joel L. Sussman