DXP reductoisomerase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
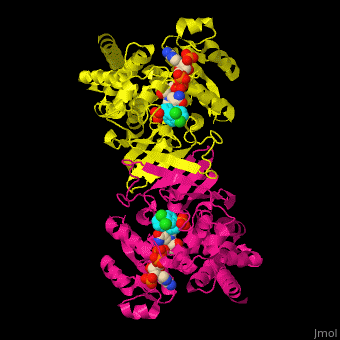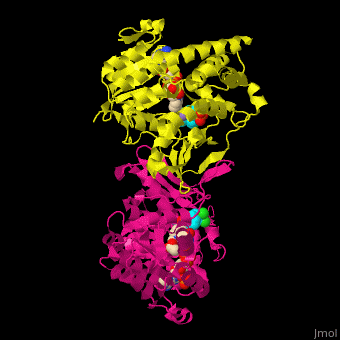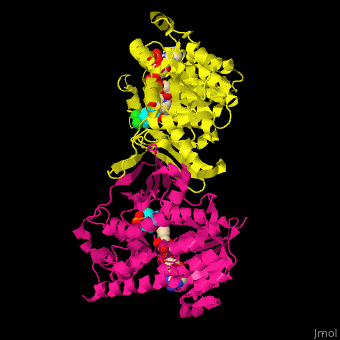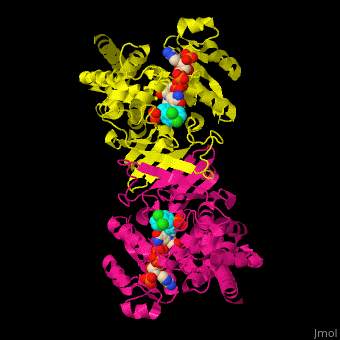
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='DXP reductoisomerase complex with NADPH, Mn+2 ion (green) and anti-malaria drug fosmidomycin (PDB code [[3zhy]])' scene='70/708083/Cv/1'> |
== Function == | == Function == | ||
'''DXP reductoisomerase''' (DXR) or '''1-deoxy-D-xylulose-5-phosphate reductoisomerase''' or '''IspC''' catalyzes the conversion of 1-deoxy-D-xylulose-5-phosphate (DXP) to 2-C-methyl-D-erythritol 4-phosphate. DXR is part of the nonmevalonate pathway which synthesizes isoprenoids which are essential components of bacterial cell wall. NADPH is a cofactor of the reaction. Divalent metal cations (Mg<sup>+2</sup>, Mn<sup>+2</sup>) are involved in binding of the DXP substrate to DXR.<ref>PMID:9707569</ref> | '''DXP reductoisomerase''' (DXR) or '''1-deoxy-D-xylulose-5-phosphate reductoisomerase''' or '''IspC''' catalyzes the conversion of 1-deoxy-D-xylulose-5-phosphate (DXP) to 2-C-methyl-D-erythritol 4-phosphate. DXR is part of the nonmevalonate pathway which synthesizes isoprenoids which are essential components of bacterial cell wall. NADPH is a cofactor of the reaction. Divalent metal cations (Mg<sup>+2</sup>, Mn<sup>+2</sup>) are involved in binding of the DXP substrate to DXR.<ref>PMID:9707569</ref> | ||
Revision as of 10:28, 20 October 2017
| |||||||||||
3D Structures of DXP reductoisomerase
Updated on 20-October-2017
References
- ↑ Takahashi S, Kuzuyama T, Watanabe H, Seto H. A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci U S A. 1998 Aug 18;95(17):9879-84. PMID:9707569
- ↑ Jansson AM, Wieckowska A, Bjorkelid C, Yahiaoui S, Sooriyaarachchi S, Lindh M, Bergfors T, Dharavath S, Desroses M, Suresh S, Andaloussi M, Nikhil R, Sreevalli S, Srinivasa BR, Larhed M, Jones TA, Karlen A, Mowbray SL. DXR inhibition by potent mono- and disubstituted fosmidomycin analogues. J Med Chem. 2013 Aug 8;56(15):6190-9. doi: 10.1021/jm4006498. Epub 2013 Jul 17. PMID:23819803 doi:http://dx.doi.org/10.1021/jm4006498