User:Brittany Allen/Sandbox 1
From Proteopedia
| Line 44: | Line 44: | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
| - | [[Image: BLAST SEQ.png | | + | [[Image: BLAST SEQ.png |600px|right|thumb| Image 5. The binding sites of TIP30 as identified using PYMOL.]] |
When analyzing the amino acid sequence of TIP30, 98% of the protein was identical to CC3 and after further analysis, TIP30 and CC3 were identified to be the same protein[1]. | When analyzing the amino acid sequence of TIP30, 98% of the protein was identical to CC3 and after further analysis, TIP30 and CC3 were identified to be the same protein[1]. | ||
| - | [[Image: BLAST2 seq.png | | + | [[Image: BLAST2 seq.png |600px|right|thumb| Image 6. The binding sites of TIP30 as identified using PYMOL.]] |
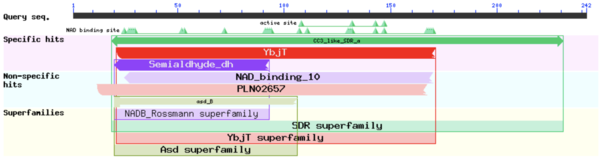
A BLAST analysis using the FASFA sequence of TIP30 from Uniprot, revealed that TIP30 has sequence similarity to SDR superfamilies. Further analysis revealed that TIP30 also contains several NAD(P) binding domains. | A BLAST analysis using the FASFA sequence of TIP30 from Uniprot, revealed that TIP30 has sequence similarity to SDR superfamilies. Further analysis revealed that TIP30 also contains several NAD(P) binding domains. | ||
Revision as of 01:35, 5 December 2017
|
Contents |
General Background Information
TIP30, also known as both CC3 [1] and HTATIP2 [2], is a tat-interacting protein that functions as a tumor suppressor involved in cell growth, apoptosis, metastasis, DNA repair, metabolism and angiogenesis of tumor cells [2,3,4]. TIP30 acts as a transcription cofactor that can regulate gene expression [5] and has both pro-apoptotic and anti-metastatic properties [6]. When the promoter of TIP30 is methylated, TIP30 becomes downregulated and associated with tumor prognosis[2]. It is thought that the tumor suppressor effect is the result of the inhibition of nuclear transport through binding with importin βs or by regulating transcription through interaction as a complex with a co-activator independent of AF-2 function and the c-myc gene[7]. Several studies have found that TIP30 may be linked to esophageal carcinoma, laryngeal carcinoma, glioma, pancreatic ductal adenocarcinoma, breast cancer, gastric cancer, gallbladder adenocarcinoma, lung cancer, and hepatocellular carcinoma[1].
Structure
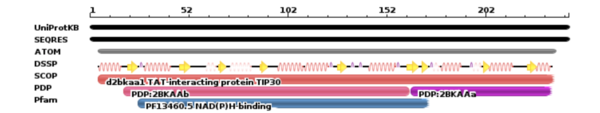
TIP30 is an evolutionary conserved gene located on human chromosome 11 [1] . TIP30 has a molecular mass of 30 kd[5] and is composed of 242 amino acids[1]. TIP30 is composed of alpha helices, beta sheets, and loops as seen in Image 1.
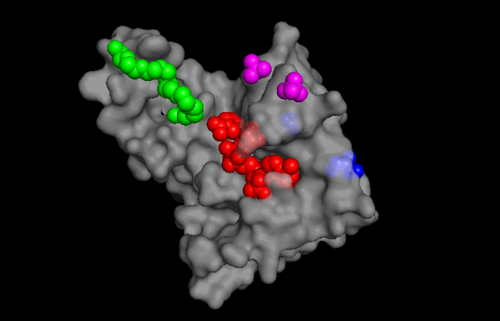
Through sequence analysis studies, it is thought that TIP30 may be a member of the SDR (substrate determining residue) family which contain a characteristic motif at their catalytic start sites [1]. The carboxyl terminus of TIP30 binds to the SDR substrate, while the amino terminus of TIP30 is the nucleotide cofactor-binding domain which has a characteristic Gly-X-X-Gly- X-X-Gly motif (where X can be any amino acid) [1]. Since SDR families have binding specificity for NADPH [8] and TIP30 contains a dehydrogenase reductase fold that contains binding specificity for NADPH [1], the binding of NADPH may be important for the biological activity of TIP30 including interactions with importins as well as the c-myc system[8]. TIP30 is known to bind 4 ligands: NDP (NADPH Dihydro-nicotinamide-adenine-dinucleotide-phosphate), PE8 (3,6,9,12,15,18,21 heptatricosane-1,2,3-diol), GOL (glycerol), and SO4 (sulfate ion)[9]. The location these residues bind can be seen in image 2. According to Uniprot, TIP30 contains a nucleotide binding region between residues 19-52, as shown in image 2. and a binding site at residue 131. When comparing images 2 and 3, it can be observed that the ligands bind in the active regions and interact with the residues. Uniprot also noted that TIP30 can contain a mutagenic site at positions 28-31 (image 4), if this site is present there is a loss of proapoptotic and metastasis-inhibiting effects.Function
TIP30 is found in both the cell nucleus and cytoplasm[10] and functions in a variety of cellular processes including apoptosis, proliferation, metastasis, angiogenesis, DNA damage repair, and metabolic adaptation.[1]In normal cell growth, TIP30 regulates DNA replication and repair. Stress activates TIP30 to regulate cell proliferation through cell cycle arrest, senescence, and apoptosis to prevent tumor formation. In this manner, TIP30 can act as a tumor-suppressor gene of Type I (caretaker) or a Type 2 (gatekeeper) to prevent mutagenesis[1]. Type I tumor-suppressor genes work by repairing damaged DNA while Type 2 halt the cell cycle to allow time to fix the DNA[1].
TIP30 Tumor Suppressive Functions
TIP30 regulates apoptosis
TIP30 has proapoptotic activity. In certain types of cancer, TIP30 will be overexpressed and cause the cells to undergo apoptosis[1]. TIP30 can also enhance the expression of p53, another tumor suppressor that can inhibit cell growth[1].
TIP30 regulates proliferation
In a study done with HCC cells, when TIP30 is expressed with increased levels of p53, and decreased levels of Bcl-2, and combined with cytotoxic drug 5-fluorouracil treatment, tumor growth was suppressed, implicating TIP30 to play a role in suppressing tumor proliferation[1].
TIP30 regulates metastasis
TIP30 can inhibit metastasis through two mechanisms: one is through an apoptosis-inducing effect, and second through an angiogenesis-inhibiting effect[1].
TIP30 regulates angiogenesis
In vitro experiments have demonstrated that TIP30 can induce changes in RNA levels of angiogenic-modulating factors that reduce angiogenesis[1]. In an in vitro experiment using both macro and microvascular origin tumor cells, angiogenic stimulator angiopoietin I was reduced while angiogenic inhibitors had increased expression, indicating that TIP30 uses angiogenic modulators to inhibit angiogenesis[1].
TIP30 controls gene expression
TIP30 is a gene transcription factor that uses tat proteins and RNA polymerase II[1]. TIP30 can mediate tumor suppressive regulation by directly affecting the transcription of genes in the nucleus or by indirectly changing the signal transduction pathways in the cytoplasm or inhibiting nuclear import of proteins[1]. TIP30 also plays a role in the regulation of EGFR, a protein that plays a role in the pathogenesis of human cancers[1].
TIP30 regulates tumor cell metabolism
In an experiment with silenced TIP30 in HeLa cells, the researchers were able to demonstrate that in the silence of TIP30, tumor cells can thrive in a low glucose setting[1]. When TIP30 is absent, mitochondrial oxidative phosphorylation of glucose is low[1]. The silencing of TIP30 in tumor cells is dependent on glycolysis to give the cells flexibility to use both OXPHOS and glycolysis for metabolism[1].
TIP30 regulates DNA damage responses
Role in Disease
TIP30 is naturally expressed in human tissues including the heart, brain, lung, kidney, and pancreas, however in cancers including breast cancer, lung cancer, liver cancer, gastric cancer, and colorectal cancer TIP30 expression is decreased. As shown above, many of the functions of TIP30 play a critical role in the prevention of disease through the control of apoptosis, growth, metastasis, angiogenesis, DNA repair, and tumor cell metabolism. When TIP30 is properly expressed, patients have a good prognosis[2],but when the TIP30 promoter becomes methylated[2,4], the loss or decrease in expression of TIP30 can contribute to irregular cell growth and development and cause tumor development[1]. For example, high levels of TIP30 can induce apoptosis while the down regulation of TIP30 in tumor cells leads to the development of tumors[3].
A recent study looked into the expression of TIP30 in patients with cancer. Over 14 studies, including 1705 patients, an association was observed that linked TIP30 expression to the good overall survival of the cancer patients[5]. Due to this study, along with previous research, promoting the expression of TIP30 is a solid potential for drug targets because of TIP30 is expressed and not down regulated, you could prevent one source of forming tumors[5].
Evolutionary Conservation
When analyzing the amino acid sequence of TIP30, 98% of the protein was identical to CC3 and after further analysis, TIP30 and CC3 were identified to be the same protein[1].
A BLAST analysis using the FASFA sequence of TIP30 from Uniprot, revealed that TIP30 has sequence similarity to SDR superfamilies. Further analysis revealed that TIP30 also contains several NAD(P) binding domains.
References
1.Yu X, Li Z, Wu WKK. TIP30: A Novel Tumor-Suppressor Gene. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics [Internet]. 2015;22(5):339–48. Available from: http://www.ingentaconnect.com/content/cog/or/2015/00000022/F0020005/art00013#
2.Xu T, Jin Z, Yuan Y, Zheng H, Li C, Hou W, et al. Tat-Interacting Protein 30 (TIP30) Expression Serves as a New Biomarker for Tumor Prognosis: A Systematic Review and Meta-Analysis. Plos One [Internet]. 2016;11(12). Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0168408
3.Hu Y, Chen F, Liu F, Liu X, Huang N, Cai X, et al. Overexpression of TIP30 inhibits the growth and invasion of glioma cells. Molecular Medicine Reports [Internet]. 2015;13(1):605–12. Available from: https://www.spandidos-publications.com/mmr/13/1/605?text=fulltext
4.Fong S, King F, Shtivelman E. CC3/TIP30 affects DNA damage repair. BMC Cell Biology [Internet]. 2010;11(1):23. Available from: https://bmccellbiol.biomedcentral.com/articles/10.1186/1471-2121-11-23
5.Zhang X, Lv L, Ouyang X, Zhang S, Fang J, Cai L, et al. Association of TIP30 expression and prognosis of hepatocellular carcinoma in patients with HBV infection. Cancer Medicine [Internet]. 2016;5(9):2180–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5055146/
6.Omari KE, Bird LE, Nichols CE, Ren J, Stammers DK. Crystal Structure of CC3 (TIP30). Journal of Biological Chemistry [Internet]. 2005;280(18):18229–36. Available from: http://www.jbc.org/content/280/18/18229.long
7.Jörnvall H. Medium- and short-chain dehydrogenase/reductase gene and protein families. Cellular and Molecular Life Sciences [Internet]. 2008;65(24):3873–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2792337/
8.K EO, LE B, CE N, J R, DK S. K [Internet]. PDB 2bka structure summary ‹ Protein Data Bank in Europe (PDBe) ‹ EMBL-EBI. 1970 [cited 2017Dec4]. Available from: https://www.ebi.ac.uk/pdbe/entry/pdb/2BKA
9.European Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics. Oxidoreductase HTATIP2 [Internet]. HTATIP2 - Oxidoreductase HTATIP2 - Homo sapiens (Human) - HTATIP2 gene & protein. 2017 [cited 2017Dec4]. Available from: http://www.uniprot.org/uniprot/Q9BUP3
10.King FW, Shtivelman E. Inhibition of Nuclear Import by the Proapoptotic Protein CC3. Molecular and Cellular Biology [Internet]. 2004;24(16):7091–101. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15282309
![Image 1. The secondary structures found in TIP30 as seen on PDB[8].](/wiki/images/thumb/a/a3/PDBstructure.png/700px-PDBstructure.png)