We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
VprBP
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> == Function == '''VPRBP''' (VPR-binding protein) is a HIV-1 WD40 protein is essentia...) |
|||
| Line 1: | Line 1: | ||
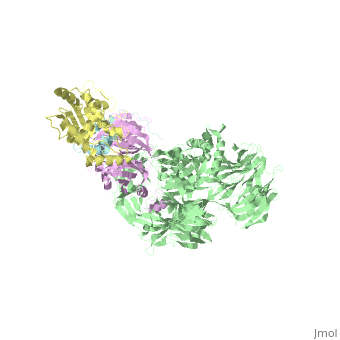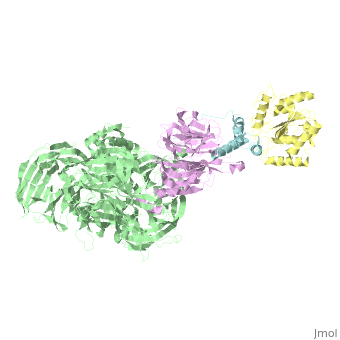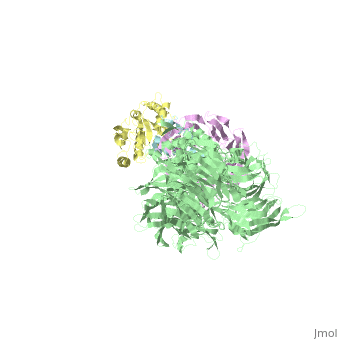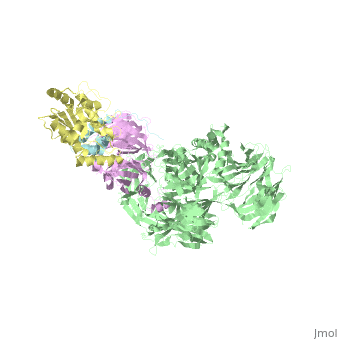
| - | + | <StructureSection load='5jk7' size='340' side='right' caption='Human VprBP residues 1045-1396 (pink) complex with Vpr (cyan), DNA damage-binding protein (grey) and uracil-DNA glycosylase (yellow) (PDB code [[5jk7]]' scene=''> | |
| - | <StructureSection load=' | + | |
== Function == | == Function == | ||
| - | ''' | + | '''VprBP''' (VPR-binding protein) is a HIV-1 WD40 protein is essential for DNA replication and embryonic development<ref>PMID:18606781</ref>. VprBP has intrinsic kinase activity and is capable of phosphorylating histone H2A. Recruitment of VprBP by Vpr is essential for HIV-1 VPR activity of initiating the host cell response cycle arresting its G(2) phase following mitosis<ref>PMID:17314515</ref>. VprBP interacts with merlin which is then recruited to the E3 ligase complex resulting in its polyubiquitination and consequently its proteasome-mediated degradation<ref>PMID:18332868</ref>. |
| - | + | ||
== Relevance == | == Relevance == | ||
| - | + | VprBP inhibition could be a strategy for the development of anticancer therapeutics<ref>PMID:24140421</ref>. | |
== Structural highlights == | == Structural highlights == | ||
| Line 19: | Line 17: | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | [[4pxw]] – | + | [[4pxw]] – hVprBP residues 1039-1401 (mutant) - human <br /> |
| - | [[3wa0]] – | + | [[3wa0]] – hVprBP residues 1417-1506 + merlin <br /> |
| - | [[4p7i]] – | + | [[4p7i]] – hVprBP residues 998-1058 + merlin <br /> |
| - | [[4z8l]], [[5aja]], [[4cc9]] – | + | [[4z8l]], [[5aja]], [[4cc9]] – hVprBP residues 1057-1396 + VPX + SAMHD1 <br /> |
| - | [[5jk7]] – | + | [[5jk7]] – hVprBP residues 1045-1396 + Vpr + DNA damage-binding protein + uracil-DNA glycosylase <br /> |
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 08:52, 19 December 2017
| |||||||||||
3D Structures of VPRBP
Updated on 19-December-2017
4pxw – hVprBP residues 1039-1401 (mutant) - human
3wa0 – hVprBP residues 1417-1506 + merlin
4p7i – hVprBP residues 998-1058 + merlin
4z8l, 5aja, 4cc9 – hVprBP residues 1057-1396 + VPX + SAMHD1
5jk7 – hVprBP residues 1045-1396 + Vpr + DNA damage-binding protein + uracil-DNA glycosylase
References
- ↑ McCall CM, Miliani de Marval PL, Chastain PD 2nd, Jackson SC, He YJ, Kotake Y, Cook JG, Xiong Y. Human immunodeficiency virus type 1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol Cell Biol. 2008 Sep;28(18):5621-33. doi: 10.1128/MCB.00232-08. Epub 2008 Jul , 7. PMID:18606781 doi:http://dx.doi.org/10.1128/MCB.00232-08
- ↑ Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, Margottin-Goguet F. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle. 2007 Jan 15;6(2):182-8. Epub 2007 Jan 17. PMID:17314515
- ↑ Huang J, Chen J. VprBP targets Merlin to the Roc1-Cul4A-DDB1 E3 ligase complex for degradation. Oncogene. 2008 Jul 3;27(29):4056-64. Epub 2008 Mar 10. PMID:18332868 doi:onc200844
- ↑ Kim K, Kim JM, Kim JS, Choi J, Lee YS, Neamati N, Song JS, Heo K, An W. VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol Cell. 2013 Nov 7;52(3):459-67. doi: 10.1016/j.molcel.2013.09.017. Epub 2013, Oct 17. PMID:24140421 doi:http://dx.doi.org/10.1016/j.molcel.2013.09.017