Introduction
Human immunodeficiency virus attacks the immune system by destroying CD4+ T cells, white blood cells that protect the body from infection. During HIV’s initial attack, it attaches to CD4 receptor cells injecting its RNA genetic material. The enzyme reverse transcriptase converts its’ RNA into DNA allowing HIV to use the CD4 cell’s machinery to replicate itself and travel through the body. As the virus attacks these cells, the immune system becomes weaker so the body is unable to fight infection, leading to the development of AIDS. Although HIV can be treated through the use of antiretroviral therapy there is currently no cure [1]. HIV’s vast genetic variability makes treatment difficult. This variability is due to the high mutation and recombination rates of the reverse transcriptase enzyme causing HIV viral sequences to differ by up to 10% in each individual [2]. An estimated 36.9 million people were suffering from HIV around the world in 2014 [3].
The Origin of HIV
There are two types of HIV known, HIV-1 and HIV-2, with HIV-1 being by far the most common and most infectious of the two strains. Research has shown that HIV-1 is closely related to the SIV virus (simian immunodeficiency virus) that attacks the immune system of chimpanzees and HIV-2 is closely related to a strain of SIV that infects sooty mangabeys.
Chimpanzees became infected with 2 different strains of SIV after eating two smaller species of monkeys (red-capped mangabeys and spot-nosed monkeys). These two strains joined together forming a third strain known as SIVcpz that could be passed on to other chimpanzees as well as to humans. It is believed that this virus was transmitted into humans as a result of them consuming chimpanzee meat or being exposed to their blood. Once inside its new host the virus adapted into what we know as HIV-1. Each time the virus was transferred from chimpanzee to human it developed slightly differently leading to the four strains of HIV-1 (M,N,O, and P). HIV-2 was transferred from sooty mangabeys to humans in a similar way. It is far less infectious and is mainly found among people in a few West African countries (Mali, Mauritania, Nigeria, and Sierra Leone)[4] .
The first known case of HIV-1 was detected in a man from Kinshasa of the Democratic Republic of the Congo during the 1950’s [5].This area is known to have the most genetic diversity among HIV strains, indicating that there were several different instances of SIV being transferred to humans. People first became aware of HIV and AIDS when it began emerging in the United States and it wasn’t until this time that it became recognized as a medical condition. [4].
Components
The different components that make up the Human Immunodeficiency Virus include viral enzymes, structural, and accessory proteins. There are three viral enzymes: Reverse Transcriptase (RT), Integrase (IN), and HIV Protease (PR). The Reverse Transcriptase builds a new DNA from the viral RNA genome aiding in its replication. Integrase is responsible for inserting a viral DNA copy into the infected cellular genome. The HIV protease is needed for maturation. The structural proteins that make up HIV are matrix, capsid, envelope, and nucleocapsid proteins. The matrix proteins are responsible for forming a coat on the viral inner membrane. The capsid proteins form a cone-shaped coat that aid the virus in being injected into cells. The envelope proteins, SU and TM, binds to the receptors of virally-infected cell to inject it with the HIV’s RNA. The nucleocapsid proteins form a complex to protect the viral RNA.
The accessory proteins include viral protein u (Vpu), viral infectivity factor (Vif), viral protein r (Vpr), P6, negative regulatory factor (Nef), regulator of virion (Rev), and trans-activator of transcription (Tat). Vpu aids in the release of virions by allowing the cell envelope to open easier, thereby enhancing the release of the new virions into the host. This is achieved by decreasing the strength of the interactions with the proteins that hold the envelopes to the cell membrane (Guatelli 2009). Viral infectivity factor (Vif) helps with the infection by marking APOBEC3G, a host protein that inhibits the virus’s virulence, to be degraded by the host’s own immune system. This makes the virus more virulent to the host. Vif is mandatory for infection in certain, but not all, types of cells[6]. Viral protein R is yet another protein that increases the infectivity of HIV. Vpr has several important functions the first of these being its ability to allow viral components to cross the nuclear membrane. Vpr is also capable of preventing host cell division by arresting cell growth in G2 phase. A separate property that this protein has been shown to possess is the ability to cause apoptosis in human cells [7]. P6 serves as a docking site for both viral and cellular materials. This protein is also necessary for Vpr to be incorporated into virions, as well as contributing to viral budding [8]. Negative regulatory factor (Nef) is important in early development of the virus. It enables T cell activation and causes the endurance of the virus. This protein downregulates the immune response of the host and its production of surface molecules, allowing the infected cells to persist [9]. Regulator of virion (Rev) interacts with viral RNA during a later stage of viral replication. It mainly functions to transport viral, unspliced RNA out of the nucleus and then ensures that these unspliced RNA are assembled into the virion particles [10]. Trans-activator of transcription (Tat) is an early HIV protein that promotes transcription by interacting with the viral long terminal repeat (LTR) at the trans-activator response element (TAR) hairpin. While its major function involves stimulating transcription, Tat regulates gene expression in other significant ways as well, such as recruiting proteins that deal with elongation and the processivity of RNA polymerase II, RNA processing, and chromatin modifications [11]
Accessory Proteins
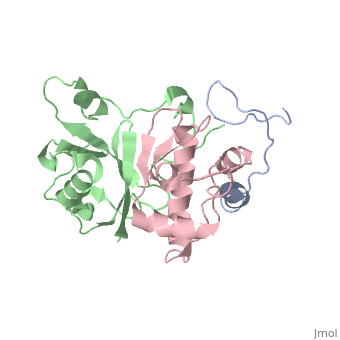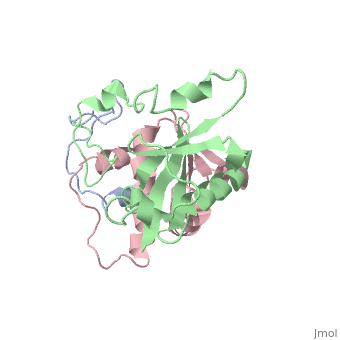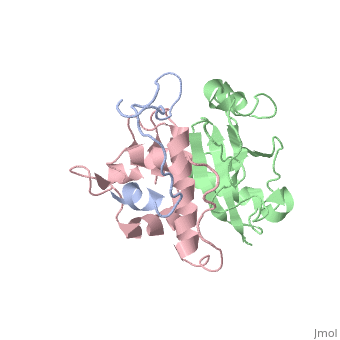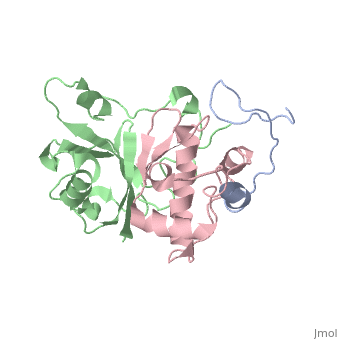
Viral infectivity factor (Vif)
(Protein Data Bank ID: 2ma9).
Viral infectivity factor (Vif) is an accessory protein with a complex, cell-specific role in the pathway of HIV. In typically non-permissible cell types, the normal targets of HIV (lymphocytes, macrophages, and some types of T-cells), Vif interacts with the cell immunity mechanism factor APOBEC3G. APOBEC3G functions by becoming incorporated into the virions, and preventing reverse transcription from occurring. Vif counteracts this by binding to APOBEC3G and tagging it to be destroyed by polyubiquitination. Vif’s functionality makes it unnecessary in permissible cell types where APOBEC3G is not present.
Vif is made up of at least two crucial domains, the N terminus of the protein which binds to APOBEC3G, and The C terminal region which contains many hydrophobic residues and is essential for the degradation of APOBEC3G. The complex workings of this protein led to the determination of its functionality taking a long time. The elucidation of this function could lead to the possible development of drugs to combat HIV [6].
Viral protein R (Vpr)
(Protein Data Bank ID: 1bde).
Viral protein R (Vpr) is among the most recent accessory proteins to be somewhat well understood. Vpr is a nucleic acid binding protein, and the presence of Vpr leads to several different outcomes in the cell.
Vpr can facilitate the movement of HIV cDNA from the cytoplasm to the nucleus where it can be utilized by the cell. Another function of Vpr is the ability to activate the transcription of HIV promoters. Vpr also possesses the ability to halt cell division between the growth 2 phase and mitosis. This activity is correlated with a decrease in the activity of p34cdc2, a crucial element for the progression from G2 to mitosis. After this halt in their growth cycle the affected cells undergo apoptosis [12].
Viral protein U (Vpu)
(Protein Data Bank ID: 1pi8).
Viral protein U (Vpu) is another accessory protein that functions in increasing the virulence of HIV-1.
How Vpu works is not completely understood but it is believed to aid in the release of virions by causing the tethering proteins to release the virions on the outside of the cell. When Vpu is not present, the virions accumulate on the outside of the cell and are not released, this is why it is believed that they cause the release of virions from the infected cell.
HIV-1 Protease (P6)
Much about P6 is still being studied. It has been discovered that P6 is very important for virion release from the cell by aiding in the budding process [13]. P6 is usually located near the cytoplasmic membrane due to its hydrophobic regions; this is not surprising given its role in virion release [8].
Negative regulatory factor (Nef)
(Protein Data Bank: 3rea).
Negative regulatory factor (Nef) performs two functions in the host cell. The first is that it is involved in T cell activation. The second is that it maintains a persistent state of infection.
Nef downregulates the expression of surface proteins important to the host immune response, such as the major-histocompatibility complexes and CD4 and CD28 on helper T cells [9].
Regulator of virion (Rev)
(Protein Data Bank: 4pmi).
Regulator of virion (Rev) functions later in the pathway of HIV. It exports unspliced RNA out of the nucleus and into the cytoplasm. This unspliced RNA is thus ensured to be incorporated during assembly of the virion particle.
Rev binds to viral RNA factor, Rev response element (RRE), available on most unspliced transcripts. Rev then escorts these unspliced transcripts out of the nucleus, utilizing the Crm1 pathway. Once in the cytoplasm, these transcripts either become the template for translation, or they are assembled into virion particles [10].
Trans-activator of transcription (Tat)
(Protein Data Bank ID: 3mia).
One way HIV regulates transcription and gene expression is through the trans-activator of transcription (Tat).
Tat acts early on in the HIV cycle. It functions to activate transcription by interacting with the long terminal repeat located in the trans-activator response (TAR) hairpin. Tat binding at this region promotes the binding of transcription factor, pTEFb, which can affect RNA polymerase II processivity and formation of transcription complexes. Tat has many other proposed functions, including recruitment of proteins involved in chromatin-remodeling, gene regulation, RNA processing, translation, and reverse transcription [11].
Pathway
During early infection, HIV is macrophage tropic, meaning that it only infects natural killer cells, CD8+ killer T cells, macrophages, cells of the nervous system, and dendritic cells. During that phase, the cells harbor, replicate, and bud HIV without lysis. On the contrary, during later infections, HIV is T-lymphocyte tropic, meaning it infects CD4+ T lymphocytes and will lyse them during production of new viruses, making it a productive infection. HIV enters a cell by interacting its gp120 portion, the head portion of the protruding glycoprotein, with CD4, the major cellular receptor that is abundantly present on T lymphocytes. This interaction causes a conformational change in gp120 and gp41, the spike portion of the protruding glycoprotein, resulting in the exposure of new binding regions on these proteins, thus causing a co-receptor reaction with either CCR5, the macrophages’ co-receptor, or fusin, the T lymphocyte co-receptor. The virus then embeds into the membrane of the CD4+ cell. The gp41 protein changes into a coiled shape that brings the virus and cell membrane close to each other, allowing the fusion of the virus’ membrane and the host cell, resulting in entry and uncoating, which is the release of viral nucleic acids. In the cytoplasm, the viral RNA is converted to DNA by the HIV-encoded reverse transcriptase. The reverse transcriptase binds to the tRNAlys, which is bound to the RNA genome that serves as a primer for the reverse transcriptase, initiating DNA synthesis. When the reverse transcription is completed, it produces a double-stranded DNA (dsDNA) that contains long terminal repeats (LTRs) located at the end of the viral genome. The viral DNA is transported to the nucleus where it may be integrated into the host’s chromosomal DNA, which is catalyzed by integrase protein found on the HIV [14].
During a productive infection, HIV-1 is reactivated and the lytic cell cycle occurs. The host’s transcriptional and translational machinery is used. The proviral DNA is transcribed by the cellular RNA polymerase II from a single promoter in the 5’ LTR into a 9 kb viral RNA primary transcript. Trans-activator of transcription (Tat) and regulator of virion (Rev) both play a role in the regulation of viral gene expression. Tat interacts with TAR, which is located at the 5’ end of all HIV RNAs, and drastically increases the transcription rate from the HIV LTR [14]. Rev binds to the Rev response element (RRE), which is a well-conserved, 350 nucleotide element that’s located in the env coding region of the viral genome [15]. This binding facilitates the export of unspliced and incompletely spliced viral RNAs from the nucleus to the cytoplasm. Once they are in the cytoplasm, some of the full-length viral messenger RNAs are packaged into virions, whereas others are spliced within the nucleus to form mRNAs that are translated by the host’s ribosome into different viral proteins. The gag gene and a combination of both the gag and pol genes together are translated into large precursor polyproteins that are cleaved by a virus encoded protease. The env protein, gp160, is transported through the trans Golgi apparatus where it is glycosylated and cleaved by a cellular protease into gp120 and gp41 to form mature envelope proteins that are targeted to the surface of the infected cell. The gp120 and gp41 are held together by covalent bonds. Tat and other smaller HIV proteins overlap with the structural genes but are in different open reading frames. Their mRNAs are made by alternative splicing of the structural gene mRNAs and are translated. The viral RNA, gag, pol, and env proteins, the virion components of HIV, are assembled at budding sites located at the cellular membrane. Two copies of the full viral genomes that contain the primer tRNA are packed into virus particles [14].
The depletion of CD4+ helper T lymphocytes caused by HIV results in AIDS and a weakening of the immune system, permitting opportunistic infections to occur. The viral env proteins found on the surface of infected cells bind to uninfected cells causing the fusion of the plasma membrane, ultimately resulting in syncytia [14].