Human Acetylcholinesterase
From Proteopedia
| Line 1: | Line 1: | ||
<StructureSection load='' size='450' side='right' scene='49/497015/Cv/1' caption='Structure of human AChE (PDB code [[4ey4]])'> | <StructureSection load='' size='450' side='right' scene='49/497015/Cv/1' caption='Structure of human AChE (PDB code [[4ey4]])'> | ||
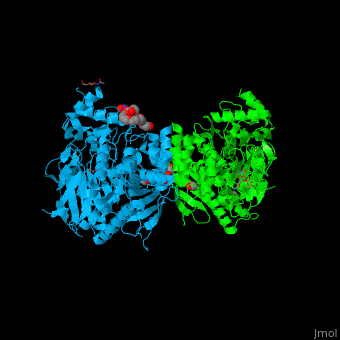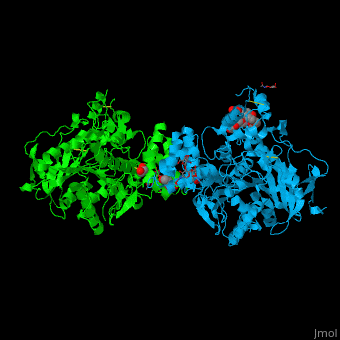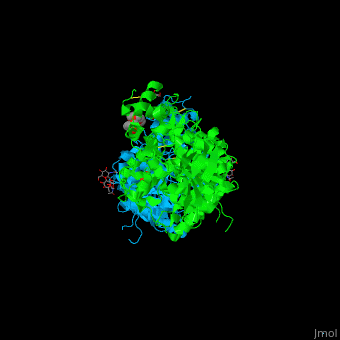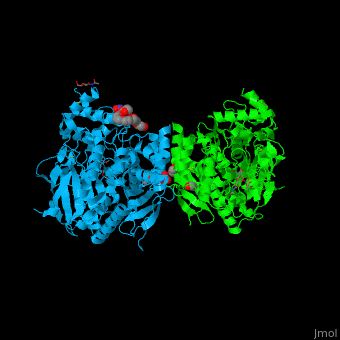
The human acetylcholinesterase (AChE) is an enzyme which hydrolyses the neurotransmitter acetylcholine (ACh) in the neuromuscular junctions and in other cholinergic synapses to terminate the neuronal signal. | The human acetylcholinesterase (AChE) is an enzyme which hydrolyses the neurotransmitter acetylcholine (ACh) in the neuromuscular junctions and in other cholinergic synapses to terminate the neuronal signal. | ||
| - | It has an ellipsoidal shape with dimensions ~ | + | It has an ellipsoidal shape with dimensions ~ 45Å x 60Å x 65Å. This protein is composed of 531 residues. It consists of 12-stranded, central mixed <scene name='49/497015/Cv/2'>β-sheet</scene> surrounded by 14 <scene name='49/497015/Cv/3'>α helices</scene>. It is a member of the α/β hydrolase fold family<ref>PMID 1409539</ref>. |
In the physiological conditions, AChE exists as tetramers associated with either collagen-like Q subunit (ColQ) or proline-rich membrane-anchoring protein (PRiMA). The AChE is linked with these anchoring molecules by a "tryptophan amphiphilic tetramerization" domain (WAT). There is also a monomeric form which is soluble in the blood. | In the physiological conditions, AChE exists as tetramers associated with either collagen-like Q subunit (ColQ) or proline-rich membrane-anchoring protein (PRiMA). The AChE is linked with these anchoring molecules by a "tryptophan amphiphilic tetramerization" domain (WAT). There is also a monomeric form which is soluble in the blood. | ||
Revision as of 19:23, 16 January 2018
| |||||||||||
References, for further information on Acetylcholinesterase
- ↑ Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al.. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197-211. PMID:1409539
- ↑ Goldenzweig A, Goldsmith M, Hill SE, Gertman O, Laurino P, Ashani Y, Dym O, Unger T, Albeck S, Prilusky J, Lieberman RL, Aharoni A, Silman I, Sussman JL, Tawfik DS, Fleishman SJ. Automated Structure- and Sequence-Based Design of Proteins for High Bacterial Expression and Stability. Mol Cell. 2016 Jul 21;63(2):337-346. doi: 10.1016/j.molcel.2016.06.012. Epub 2016, Jul 14. PMID:27425410 doi:http://dx.doi.org/10.1016/j.molcel.2016.06.012
To the structures used here:
- Li W, Mak M, Jiang H, Wang Q, Pang Y, Chen K & Han Y (2009) "Novel anti-Alzheimer's dimer Bis(7)-cognitin: cellular and molecular mechanisms of neuroprotection through multiple targets", Neurotherapeutics, vol.6, p.187-201.
- Zhang D & McCammon JA (2005) "The association of tetrameric acetylcholinesterase with colQ tail: a block normal mode analysis", PLoS Comput Biology, vol.1, p.484-491.
To the active site of acetylcholinesterase
- Rosenberry TL (2009) "Strategies to resolve the catalytic mechanism of acetylcholinesterase", Journal of Molecular Neuroscience.
- Currently (November 05, 2009), part of the content of this page is inspired from a source: http://www.biochimie.univ-montp2.fr/licence/enzymo/ache/ache.htm
Hélène ERASIMUS, Blandine FAUVEL, Tiphaine Jaeg 17:08, 12 November 2009 (IST)