We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Adenylosuccinate Synthetase
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
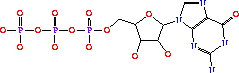
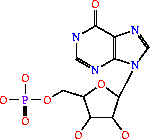
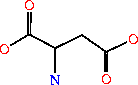
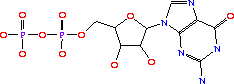
Its systematic name is IMP:L-aspartate ligase. It is mainly involved in purine bio-synthesis. It does this by catalyzing the GTP dependent changeover of IMP and aspartic acid to AMP in the presense of Mg<sup>2+</sup>.<ref>PMID:1698173 </ref> In Humans, AdSS catalyzes the first committed step in the purine nucleotide cycle by the de novo synthesis of adenosine monophosphate.<ref>PMID:649264</ref> | Its systematic name is IMP:L-aspartate ligase. It is mainly involved in purine bio-synthesis. It does this by catalyzing the GTP dependent changeover of IMP and aspartic acid to AMP in the presense of Mg<sup>2+</sup>.<ref>PMID:1698173 </ref> In Humans, AdSS catalyzes the first committed step in the purine nucleotide cycle by the de novo synthesis of adenosine monophosphate.<ref>PMID:649264</ref> | ||
| - | AdSS (3hid) was isolated from [http://en.wikipedia.org/wiki/Yersinia_pestis <i>Yersinia pestis CO92</i>]and can be found in a variety of organisms ranging from yeast to bacteria to humans. Its crystal structure was determined using x-ray diffraction at a resolution of 1.60 Angstoms. <scene name='38/382959/Cv/3'>Ligand PG6 binding site</scene>. The gene is located on chromosome 1 q44, in humans and is expressed in the majority of an organisms cells. See also [[Ligases]]. | + | AdSS ([[3hid]]) was isolated from [http://en.wikipedia.org/wiki/Yersinia_pestis <i>Yersinia pestis CO92</i>]and can be found in a variety of organisms ranging from yeast to bacteria to humans. Its crystal structure was determined using x-ray diffraction at a resolution of 1.60 Angstoms. <scene name='38/382959/Cv/3'>Ligand PG6 binding site</scene>. The gene is located on chromosome 1 q44, in humans and is expressed in the majority of an organisms cells. See also [[Ligases]]. |
==Structure== | ==Structure== | ||
Revision as of 14:54, 22 February 2018
| |||||||||||
3D structures of Adenylosuccinate Synthetase
Updated on 22-February-2018
References
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=3HID
- ↑ Mukhopadhyay RP, Chandra AL. Keratinase of a streptomycete. Indian J Exp Biol. 1990 Jun;28(6):575-7. PMID:1698173
- ↑ Pierloot RA. The treatment of psychosomatic disorders by the general practitioner. Int J Psychiatry Med. 1977-1978;8(1):43-51. PMID:649264
- ↑ 4.0 4.1 Poland BW, Silva MM, Serra MA, Cho Y, Kim KH, Harris EM, Honzatko RB. Crystal structure of adenylosuccinate synthetase from Escherichia coli. Evidence for convergent evolution of GTP-binding domains. J Biol Chem. 1993 Dec 5;268(34):25334-42. PMID:8244965
- ↑ Ware JD, Bellini WJ, Ash RJ. Metabolic characteristics of cells infected with a herpesvirus of turkeys. J Natl Cancer Inst. 1975 Dec;55(6):1379-82. PMID:1548
- ↑ Katsarkas A, Kirkham TH. Paroxysmal positional vertigo--a study of 255 cases. J Otolaryngol. 1978 Aug;7(4):320-30. PMID:691098
- ↑ Van der Weyden MB, Kelly WN. Human adenylosuccinate synthetase. Partial purification, kinetic and regulatory properties of the enzyme from placenta. J Biol Chem. 1974 Nov 25;249(22):7282-9. PMID:4436310
- ↑ Bates PC, Millward DJ. Muscle growth and protein turnover in a fast growing rat strain. Proc Nutr Soc. 1978 May;37(1):19A. PMID:662843
- ↑ Iancu CV, Borza T, Choe JY, Fromm HJ, Honzatko RB. Recombinant mouse muscle adenylosuccinate synthetase: overexpression, kinetics, and crystal structure. J Biol Chem. 2001 Nov 9;276(45):42146-52. Epub 2001 Sep 17. PMID:11560929 doi:10.1074/jbc.M106294200
- ↑ Tyagi AK, Cooney DA. Identification of the antimetabolite of L-alanosine, L-alanosyl-5-amino-4-imidazolecarboxylic acid ribonucleotide, in tumors and assessment of its inhibition of adenylosuccinate synthetase. Cancer Res. 1980 Dec;40(12):4390-7. PMID:7438071
- ↑ Weber G. Enzymes of purine metabolism in cancer. Clin Biochem. 1983 Feb;16(1):57-63. PMID:6861338
Proteopedia Page Contributors and Editors (what is this?)
Aaron Smith, Michal Harel, Alexander Berchansky, David Canner, Andrea Gorrell