Sandbox Reserved 1329
From Proteopedia
| Line 18: | Line 18: | ||
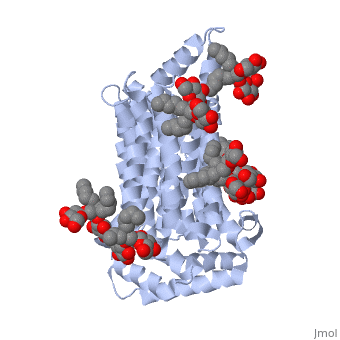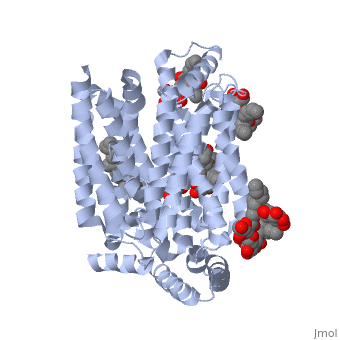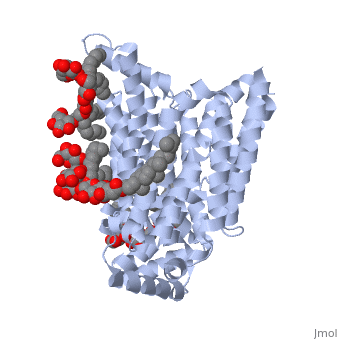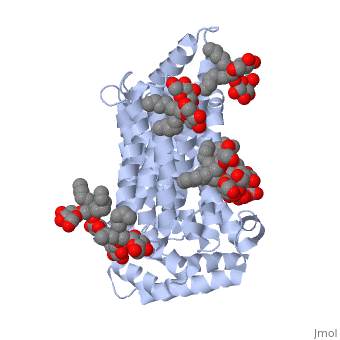
The protein contains <scene name='77/777649/Helices/1'>12 membrane-spanning alpha helices</scene> and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm. | The protein contains <scene name='77/777649/Helices/1'>12 membrane-spanning alpha helices</scene> and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm. | ||
| - | The protein also contains nine <scene name='77/777649/Ligands_37x/1'>37X ligands</scene>. The molecule itself is octyl glucose neopentyl glycol. | + | The protein also contains nine <scene name='77/777649/Ligands_37x/1'>37X ligands</scene>. The molecule itself is octyl glucose neopentyl glycol, which is part of a class of surfactants called glucose-neopentyl glycol (GNG) amphiphiles. GNG amphiphiles are very useful for solubilization and stabilization of membranes |
| - | The protein has another kind of ligand, <scene name='77/777649/Ligands_y01/1'>Y01</scene>. This molecule is cholesterol hemisuccinate. | + | The protein has another kind of ligand, <scene name='77/777649/Ligands_y01/1'>Y01</scene>. This molecule is cholesterol hemisuccinate, which is a membrane stabilizer. This is important for the function of GLUT3 as it transports glucose across the plasma membranes. |
</StructureSection> | </StructureSection> | ||
| Line 26: | Line 26: | ||
== References == | == References == | ||
Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009, October). Will the original glucose transporter isoform please stand up! Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/19690067 | Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009, October). Will the original glucose transporter isoform please stand up! Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/19690067 | ||
| + | |||
| + | Chae, P. S., Rana, R. R., Gotfryd, K., Rasmussen, S. G., Kruse, A. C., Cho, K. H., . . . Gellman, S. H. (2013, March 21). Glucose-Neopentyl Glycol (GNG) Amphiphiles for Membrane Protein Solubilization, Stabilization and Crystallization. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578972/ | ||
Cheeseman, C., & Long, W. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 167. doi:10.2147/chc.s60484 | Cheeseman, C., & Long, W. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 167. doi:10.2147/chc.s60484 | ||
| + | |||
| + | Ding, W. X., Qi, X. R., Li, P., Maitani, Y., & Nagai, T. (2005, August 26). Cholesteryl hemisuccinate as a membrane stabilizer in dipalmitoylphosphatidylcholine liposomes containing saikosaponin-d. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/15978754 | ||
Mueckler, M., & Thorens, B. (2013). Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ | Mueckler, M., & Thorens, B. (2013). Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ | ||
Potaman, V. N. (1970, January 01). DNA: Alternative Conformations and Biology. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK6545/ | Potaman, V. N. (1970, January 01). DNA: Alternative Conformations and Biology. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK6545/ | ||
Revision as of 20:28, 27 February 2018
| This Sandbox is Reserved from January through July 31, 2018 for use in the course HLSC322: Principles of Genetics and Genomics taught by Genevieve Houston-Ludlam at the University of Maryland, College Park, USA. This reservation includes Sandbox Reserved 1311 through Sandbox Reserved 1430. |
To get started:
More help: Help:Editing |
Human Glucose Transporter GLUT3/SLC2A3
| |||||||||||
References
Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009, October). Will the original glucose transporter isoform please stand up! Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/19690067
Chae, P. S., Rana, R. R., Gotfryd, K., Rasmussen, S. G., Kruse, A. C., Cho, K. H., . . . Gellman, S. H. (2013, March 21). Glucose-Neopentyl Glycol (GNG) Amphiphiles for Membrane Protein Solubilization, Stabilization and Crystallization. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578972/
Cheeseman, C., & Long, W. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 167. doi:10.2147/chc.s60484
Ding, W. X., Qi, X. R., Li, P., Maitani, Y., & Nagai, T. (2005, August 26). Cholesteryl hemisuccinate as a membrane stabilizer in dipalmitoylphosphatidylcholine liposomes containing saikosaponin-d. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/15978754
Mueckler, M., & Thorens, B. (2013). Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/
Potaman, V. N. (1970, January 01). DNA: Alternative Conformations and Biology. Retrieved February 27, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK6545/