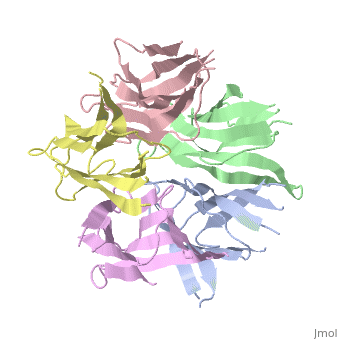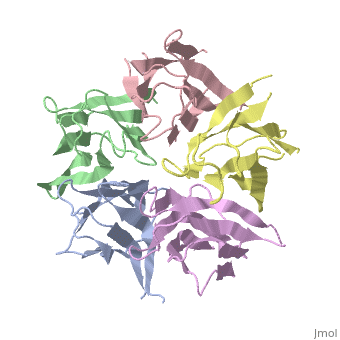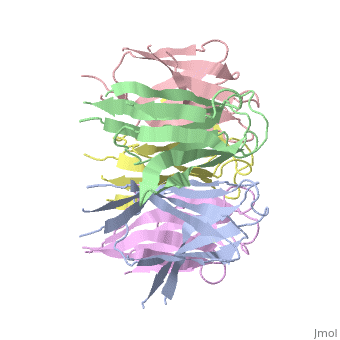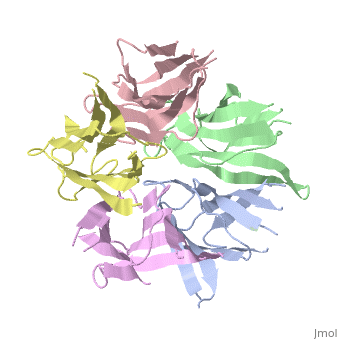We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Nucleoplasmin
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
the NP in Humans is made out of <scene name='46/467273/Dimer/1'>dimer</scene> while each <scene name='46/467273/Monomer/2'>monomer</scene> consisted of five chains. The structure remain similiar to the one in Xenopus but with a change in amino acids in its <scene name='46/467273/Core_domain/2'>core domain</scene>, Val insted of Ile. | the NP in Humans is made out of <scene name='46/467273/Dimer/1'>dimer</scene> while each <scene name='46/467273/Monomer/2'>monomer</scene> consisted of five chains. The structure remain similiar to the one in Xenopus but with a change in amino acids in its <scene name='46/467273/Core_domain/2'>core domain</scene>, Val insted of Ile. | ||
The Decamer bind H2A-H2B dimers and H3-H4 tetramers simultaneously, In the absence of histone tetramers the pentamer binds H2A-H2B and formes central hub. When H3-H4 tetramers are recruited this results in a functional dimerization of the complex, and the decamer being formed. | The Decamer bind H2A-H2B dimers and H3-H4 tetramers simultaneously, In the absence of histone tetramers the pentamer binds H2A-H2B and formes central hub. When H3-H4 tetramers are recruited this results in a functional dimerization of the complex, and the decamer being formed. | ||
| + | |||
| + | == conservastion == | ||
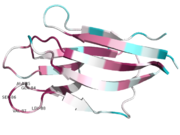
| + | The loop in the core domain of Xenopus NP is not highly conserved, [[Image:1k5j.png | thumb | Xenopus]] | ||
| + | while the core domain in human is more conserved compared to Xenopus. [[Image:3T30.png | thumb | Human]] | ||
| + | [[Image:1-s2.0-S0022519316001053-gr5.jpg]] | ||
| + | |||
| + | The general structure remain similiar, even with changes in amino acids in the core domains between the two species. That can point to the fact they have similar roles as a molecular chaperone in Human and Xenopus. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
==3D structures of nucleoplasmin== | ==3D structures of nucleoplasmin== | ||
| Line 35: | Line 59: | ||
4. SHU Te-Jun, ZHANG Yao-Zhou. Nucleoplasmin, an Important Nuclear Chaperone. Chinese Journal of Biochemistry and Molecular Biol. 15 March 2007 http://cjbmb.bjmu.edu.cn/EN/Y2007/V23/I09/718 | 4. SHU Te-Jun, ZHANG Yao-Zhou. Nucleoplasmin, an Important Nuclear Chaperone. Chinese Journal of Biochemistry and Molecular Biol. 15 March 2007 http://cjbmb.bjmu.edu.cn/EN/Y2007/V23/I09/718 | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
| + | |||
| + | 5. Platonova, O., Akey, I.V., Head, J.F., Akey, C.W.Crystal structure and function of human nucleoplasmin (npm2): a histone chaperone in oocytes and embryos.Biochemistry.2011 Sep 20;50(37):8078-89. | ||
| + | PMCID:PMC3172369 DOI:10.1021/bi2006652 | ||
Revision as of 10:40, 2 March 2018
| |||||||||||