We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Stephanie Nahhas/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
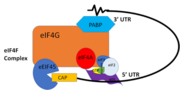
| - | PABP is a mRNA binding protein that binds to the 3’ Poly(A) tail on mRNA. Through extensive Adenosine recognition by the RRMs of PABP, the protein is involved in three main functions: recognition of the 3’ Poly(A) tail, mRNA stabilization, and eukaryotic translation initiation. The contributions of controlling gene expression via different families of PABPs is not yet fully understood. PABP families are divided into nuclear and cytoplasmic. | + | PABP is a mRNA binding protein that binds to the 3’ Poly(A) tail on mRNA. Through extensive Adenosine recognition by the RRMs of PABP, the protein is involved in three main functions: recognition of the 3’ Poly(A) tail, mRNA stabilization, and eukaryotic translation initiation. The contributions of controlling gene expression via different families of PABPs is not yet fully understood. PABP families are divided into nuclear and cytoplasmic. <ref name="Roles of Cytoplasmic Poly(A)-Binding Proteins">Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.</ref> PABP1, which is predominantly cytoplasmic, is often referred to as PABP because it is the only form of PABP that has been extensively studied in its role with mRNA translation and stability. <ref name="Roles of Cytoplasmic Poly(A)-Binding Proteins">Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.</ref> |
== Structure == | == Structure == | ||
| - | The crystal structure PABP was derived from X-ray Diffraction at 2.6Å (R-value: 23%). It is comprised of four [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motifs] (RRMs), which are highly conserved RNA-binding domains.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> The RRM in PABP is found in over two hundred families of proteins across species, indicating that it is ancient.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> RRM1 and 2 are examined in this article. Each RRM has a four-stranded antiparallel beta sheet backed by two corresponding alpha helices. <ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> mRNA poly-adenosine recognition is due to the presence of the conserved residues within the beta-sheet surface <ref name="The Poly(A)-Binding Protein and an mRNA Stability Protein Jointly Regulate an Endoribonuclease Activity.">Wang, Zuoren and Kiledjian, Megerditch. “The Poly(A)-Binding Protein and an mRNA Stability Protein Jointly Regulate an Endoribonuclease Activity.” Molecular and Cellular Biology 20.17 (2000): 6334–6341. Print.</ref> , which forms a <scene name='78/782616/Trough2/1'>trough</scene>-like pocket for the mRNA to bind. The beta-sheet flooring present in PABP interacts with the 3’ mRNA tail via a combination of van der Waals, aromatic stacking, and Hydrogen bonding. Through these interactions, PABP binds to 3’ poly-adenosine tail with a KD of 2-7 nM. | + | The crystal structure PABP was derived from X-ray Diffraction at 2.6Å (R-value: 23%). It is comprised of four [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motifs] (RRMs), which are highly conserved RNA-binding domains.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> The RRM in PABP is found in over two hundred families of proteins across species, indicating that it is ancient.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> RRM1 and 2 are examined in this article. Each RRM has a four-stranded antiparallel beta sheet backed by two corresponding alpha helices. <ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> mRNA poly-adenosine recognition is due to the presence of the conserved residues within the beta-sheet surface <ref name="The Poly(A)-Binding Protein and an mRNA Stability Protein Jointly Regulate an Endoribonuclease Activity.">Wang, Zuoren and Kiledjian, Megerditch. “The Poly(A)-Binding Protein and an mRNA Stability Protein Jointly Regulate an Endoribonuclease Activity.” Molecular and Cellular Biology 20.17 (2000): 6334–6341. Print.</ref> , which forms a <scene name='78/782616/Trough2/1'>trough</scene>-like pocket for the mRNA to bind. The beta-sheet flooring present in PABP interacts with the 3’ mRNA tail via a combination of van der Waals, aromatic stacking, and Hydrogen bonding. Through these interactions, PABP binds to 3’ poly-adenosine tail with a KD of 2-7 nM. <ref name="Roles of Cytoplasmic Poly(A)-Binding Proteins">Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.</ref> |
Further, the RRM1/2 complex interacts with the mRNA's sugar-phosphate backbone, where 4 of the 8 mRNA adenosines interact electrostatically.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> Upon closer examination of the PABP structure, the protein contains loop-like domains that form the walls of the beta-sheet trough. Although these loop walls are present, no interaction occurs between the mRNA and these regions. We propose that these loops only keep unwanted cellular elements out of the binding pocket via hydrophobic and hydrophilic interactions, maintaining the protein's selectivity for mRNA. The structural elements highlighted consist of the RRM1/2 subunits, the linker domain, and the Poly(A) mRNA binding trough. | Further, the RRM1/2 complex interacts with the mRNA's sugar-phosphate backbone, where 4 of the 8 mRNA adenosines interact electrostatically.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> Upon closer examination of the PABP structure, the protein contains loop-like domains that form the walls of the beta-sheet trough. Although these loop walls are present, no interaction occurs between the mRNA and these regions. We propose that these loops only keep unwanted cellular elements out of the binding pocket via hydrophobic and hydrophilic interactions, maintaining the protein's selectivity for mRNA. The structural elements highlighted consist of the RRM1/2 subunits, the linker domain, and the Poly(A) mRNA binding trough. | ||
| Line 73: | Line 73: | ||
===Studies on Mutations=== | ===Studies on Mutations=== | ||
| - | Studies conducted on [https://en.wikipedia.org/wiki/Drosophila Drosophila] are common due to 75% conservation between human and Drosophila genomes. Drosophila only encode one cytoplasmic PABP, and its deletion results in embryonic lethality. | + | Studies conducted on [https://en.wikipedia.org/wiki/Drosophila Drosophila] are common due to 75% conservation between human and Drosophila genomes. Drosophila only encode one cytoplasmic PABP, and its deletion results in embryonic lethality. <ref name="Roles of Cytoplasmic Poly(A)-Binding Proteins">Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.</ref> Similarly, in [https://en.wikipedia.org/wiki/Caenorhabditis_elegans Caenorhabditis elegans], which have two cytoplasmic PABPs, display 50-80% embryonic lethality with the introduction of an RNAi to one of these PABPs. <ref name="Roles of Cytoplasmic Poly(A)-Binding Proteins">Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.</ref> |
Revision as of 22:12, 2 April 2018
Human Poly(A) Binding Protein (1CVJ)
| |||||||||||
References
- ↑ Blobel, Gunter. “A Protein of Molecular Weight 78,000 Bound to the Polyadenylate Region of Eukaryotic Messenger Rnas.” Proceedings of the National Academy of Sciences of the United States of America, vol. 70, no. 3, 1973, pp. 924–8.
- ↑ Baer, Bradford W. and Kornberg, Roger D. "The Protein Responsible for the Repeating Structure of Cytoplasmic Poly(A)-Ribonucleoprotein." The Journal of Cell Biology, vol. 96, no. 3, Mar. 1983, pp. 717-721. EBSCOhost.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print.
- ↑ Kühn, Uwe and Elmar, Wahle. “Structure and Function of Poly(a) Binding Proteins.” Bba - Gene Structure & Expression, vol. 1678, no. 2/3, 2004.
- ↑ 5.0 5.1 5.2 5.3 5.4 Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.
- ↑ Wang, Zuoren and Kiledjian, Megerditch. “The Poly(A)-Binding Protein and an mRNA Stability Protein Jointly Regulate an Endoribonuclease Activity.” Molecular and Cellular Biology 20.17 (2000): 6334–6341. Print.