This is a default text for your page Isabelle A. Altieri/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
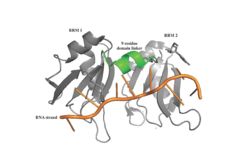
Figure 1: PABP Biological Assembly with linker highlighted.
Human Poly(A) Binding Protein (PABP) is a biopolypeptide involved in recognizing the 3' poly(A) tail of mRNA that is added to an mRNA transcript during mRNA processing.This recognition as well as PABP's interaction with other proteins and initiation factors causes it to also play a significant role in translation initiation and mRNA stabilization and degradation. PABP consists of four conserved domains of RNA recognition motifs (RRMs); however, the two N-terminal RRMs (RRM1 and RRM2) and the short linker sequence that connects them supports most of the function of PABP, so RRM3 and RRM4 may not be essential. Thus, the published X-ray structure, found by X-ray diffraction, exhibits RRM1 and RRM2 at a 2.6 Angstrom resolution. This is shown as . Both RRM 1 and 2 are needed to support biochemical function, that is, no one RRM can support biochemical function. Additionally, there is a proline rich C-terminal portion of variable length that is not well conserved and unknown as to how it contributes to the protein's function [3].
Function and Structure
Poly (A)Binding
Image:Adenosine stacking.jpg Figure 2: The specific weak intermolecular interactions between RNP1 and RNP2 and Adenosines. These interactions are the primary support of adenosine recognition by PABP and include mainly van der Waals interactions, hydrogen bonds, and stacking interactions.
A primary function of PABP is recognizing and interacting with the 3'poly (A) tail created in mRNA processing. As found by EMSA competition experiments, there is a minimum of 11-12 adenosines necessary in the poly (A) tail for the adenosine chain to bind to PABP with high affinity. However, for one biological assembly, a chain containing 9 adenosines sufficiently binds the assembly for crystallization and is shown in the biological assembly structure. The 4 RRM domains that are the primary interacting sites for the adenosine recognition exist as globular domains, each having four antiparallel β-strands and two α-helices. With the N-terminal to C-terminal motifs labeled as S1 to S4 for the β-strands and H1 to H2 for the α-helices, the strands are spatially arranged as S2-S3-S1-S4. Furthermore, there are two conserved sequences in each RRM, called RNP1 and 2. RNP 1 consists of a conserved sequence of 8 residues, while RNP2 consists of a conserved sequence of 6 residues. Much of the weak intermolecular interactions with adenosine from the RRMs occur from the conserved sequences, which correspond to the two central β-strands, with specific interactions shown in Figure 2.The support for adenosine recognition by the RRMs occurs as a type of binding trough with the sheets, primarily forming the primary binding trough, and the interstrand loop between β-strands 2 and 3 as well as the domain linker forming the . It is believed that the is disordered in the absence of RNA because it does not intramolecularly interact with either of the RRM motifs. Additionally, the primary binding trough is stabilized by . [3]
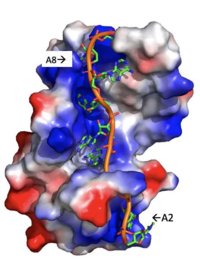
Figure 3: Basic residues of RRM 1 and 2 (shown in blue) make stabilizing electrostatic interactions with the negatively charged adenosine phosphates.
Adenosine Stabilization Interaction Patterns
Specifically, there are several significant interaction patterns that stabilize adenosine recognition. RRM 1 and 2 makes significant interactions with the adenosine backbone, shown in Figure 3. Additionally, the adenosine stabilizes itself within the binding by intramolecular stacking interactions between adenosines. Through the extensive , the RRM specifies the position of adenosine 2, allowing it to make strong intramolecular stacking interactions with adenosine 1. As a result, adenosine 1 requires less contact with the RRM, as it is mostly stabilized by adenosine 2. Furthermore, some adenosines like adenosine 3 and adenosine 6 are stabilized by being sandwiched between aromatic and alipathic side chains. occurs between aromatic and alipathic side chains and is specified by Lysine 104, and occurs similarly, but it is specified doubly by two residues, Trp-86 and Gln-88 [3].
Translation Initiation
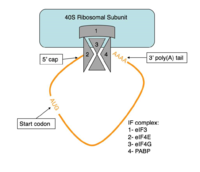
Figure 4:Closed loop model diagram required for translation initiation in eukaryotes.
The initiation of translation in eukaryotes requires many translation factors and proteins, one of which is PABP. There is evidence that PABP is critical for formation of the “closed loop” model of protein synthesis, which involves joining the 3’ poly (A) tail of mRNA to the 5’ cap to create circular RNA [4]. This process utilizes eIF4F, a protein composed of multiple translation factors that play various roles in translation. eIF4G is a scaffolding protein that binds the other subunits, eIF4E and eIF4A. eIF4E creates interactions with the 5’ cap to bring the initiation factor (IF) complex to the 5’ end of the mRNA. eIF4G also has a binding site for PABP, which is N-terminal to the binding site for eIF4F and interacts with the same RRMs that allow PABP to bind RNA. [4] All of these proteins are known to be involved in protein synthesis, but several mechanisms have been proposed for how PABP might be promoting translation.
One of the mechanisms that PABP has been shown to assist in the initiation of protein synthesis is by interaction with eIF4G. The results of Kahvejian et al. were able to show that not only might PABP be acting as a translation factor in eukaryotic cells, but it also needs to interact with eIF4G in order to have an effect.
Furthermore, PABP interactions have been shown to be critical for the formation of the 80S ribosomal subunit. PABP is essential in recruiting both the 40S and the 60S ribosomal subunit for the initiation of translation[6]. Because of this, PABP affects the formation of the 80S ribosome complex in two ways: indirectly, by allowing the 40S subunit to be available to bind, and directly, by promoting association of the 60S subunit with the 40S subunit.
In addition to these interactions, the PABP aids in this closed loop formation via interactions with Poly (A) Interacting Protein 1 (PAIP-1). In this process, PABP interacts with PAIP-1 to facilitate the complex to interact with eIF4A initiation factor. eIF4A unwinds the 5' untranslated region (UTR) of an mRNA transcript, which signals eIF4G to function as previously mentioned [3]. Thus, there are two mechanisms to initiate this loop closing pathway with eIF4G. All of these proposed interactions show that PABP stimulates translation at several points throughout the initiation process.
Structural Components of PABP Translation Initiation
The RRMs support interactions with the interacting proteins such as eIF4G and PAIP-1, however, the specific ways in which PABP interacts with these proteins are not structurally proven. However, there is a convex dorsal surface present on the RRM 1 and 2 motifs formed by the two α-helices in each RRM, specified as H1 and H2 in RRM1 and H1' and H2' in RRM2. This surface contains a sequence portion of and
residues. It is thought that this area of conservation thus produces overlapping binding sites to interact with eIF4G and PAIP-1. For example, the conserved acidic residues may be beneficial to be used in to interact with essential basic residues present in both eIF4G and PAIP-1 via ionic interactions [3]. While there are only proposed mechanisms for how PABP promotes the initiation of translation in Homo sapiens, there is a pathogenic protozoan, Leishmania, that through a study done by Osvaldo P. de Melo Neto et. al, a site on the domain linker of a PABP homolog, PABP-1, of either serine-proline or threonine-proline residues which an eIF4F-like complex phosphorylates. The authors suggest that this phosphorylation is part of how PABP-1 aids the eIF4F complex in initiating translation. They supported this by removing the gene that encodes PABP-1 and the results showed that the protozoan could not initiated cell growth and therefore survive without the PABP-1 gene. The homo sapiens' PABP also contain a () site on the domain linker which could be interacting with the eIF4 complex in a similar way as in Leishmania protozoan.[5]
Medical Relevancy
Rotavirus' Effect on Initiation of Translation
The initiation of translation in eukaryotes is supported by a closed loop model. This model requires the 5' end and the 3' end of mRNA to be physically connected. The poly(A)-binding protein is necessary for initiation of translation and is required for the closed loop model. Rotavirus, a virus of varying size, containing 11 double stranded RNA and 12 proteins (6 structural, 6 non-structural) is responsible for preventing initiation of translation in infected cells. The virus enters the cell and undergoes a non-conservative replication cycle in the cytoplasm. After a replication cycle non-structural protein 3 (NSP3) can be found spread throughout the cytoplasm. NSP3 is responsible for releasing PABP from eIF4F and inhibiting translation initiation. In a study done by Piron et al. it has been seen that NSP3 competes with PABP in binding to the poly(A)-tail of mRNA. This competitor inhibits the proper closing of the closed loop therefore inhibiting translation and protein synthesis. Not only does the rotavirus inhibit protein synthesis of the host cell but it successfully initiatives its own translation as well. The viral mRNA and the host translation initiation factors are in close enough proximity to allow the viral mRNA bound to NSP3 to undergo translation. The translation of viral mRNA allows the virus to spread throughout an organism and lead to a greater decrease in host protein synthesis. When infected with rotavirus one may experience diarrhea,fever, vomiting, and dehydration. Without an antiviral it is suggested to increase fluid intake and allow three to seven days for the infection to subside. [4]
Biological Relevancy
Poly(A) Binding Protein's Evolution in plants
PABP is a conserved protein in eukaryotes and has been identified in yeast, mammals, and plants. While PABP is seen among these three it is only present in yeast as a single protein. Mammalian and plant PABP is in a diverse gene family and may have isoforms which have more specific functions. In a study done by Gallie and Liu it was seen that Arabidopsis, a small flowering plant, and yeast have multiple distinct PABP proteins which perform specific functions. The various PABP proteins in yeast vary in functions including acceleration of mRNA into a degradation pathway, mRNA biogenesis and export, and protecting the 5’ cap from degradation. While different species have different specific proteins the RRMs in PABP are highly conserved. The PABP has evolved overtime form a single gene found in yeast and algae to a large family of genes found in land plants and mammalian species. When PABP is viewed among different species of plants including Chlamydomonas reinhardtii, Chaetosphaeridium globosum, and Klebsormidium flaccidum it has been seen that different intron patterns are conserved in different species. The evolution of PABP has been seen among water plants, bryophytes, and vascular plants as one of four classes of the gene. Class I genes are seen among certain basal angiosperms, monocots, gymnosperms, and bryophytes. Class II genes appear in seed plants while class III genes appear in specific charophytes and lycophytes. PABP evolved in plants from a single gene to two genes and eventually to a four-group gene family. This can be tracked with first marine algae then fresh water algae and finally non-vascular plants. While the divergence of PABP gene families may impact the way protein interactions the PABP proteins all have similar functions. Gallie and Liu suggest that PABP gene families are still undergoing evolution [6].
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 3.3 Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell, vol. 98, no. 6, 1999, pp. 835–845., doi:10.1016/s0092-8674(00)81517-2.
- ↑ 4.0 4.1 Piron, M. “Rotavirus RNA-Binding Protein NSP3 Interacts with eIF4GI and Evicts the Poly(A) Binding Protein from eIF4F.” The EMBO Journal, vol. 17, no. 19, 1998, pp. 5811–5821., doi:10.1093/emboj/17.19.5811.
- ↑ Cite error: Invalid
<ref> tag;
no text was provided for refs named Osvaldo
- ↑ Gallie, Daniel R, and Renyi Liu. “Phylogenetic Analysis Reveals Dynamic Evolution of the Poly(A)-Binding Protein Gene Family in Plants.” BMC Evolutionary Biology, vol. 14, no. 1, 2014, doi:10.1186/s12862-014-0238-4.



