User:David Ryskamp/Sandbox1
From Proteopedia
| Line 1: | Line 1: | ||
==Human Poly(A) Binding Protein (1CVJ)== | ==Human Poly(A) Binding Protein (1CVJ)== | ||
| - | <StructureSection load='1cvj' size='340' side='right' caption='PABP' scene='78/782614/Structure_scene_color_scheme/1'> | ||
== Background == | == Background == | ||
| Line 10: | Line 9: | ||
== Structure == | == Structure == | ||
| - | + | <StructureSection load='1cvj' size='340' side='right' caption='PABP' scene='78/782614/Structure_scene_color_scheme/1'> | |
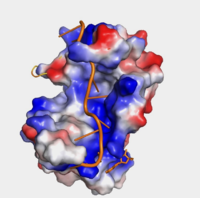
The crystal structure PABP was derived from X-ray Diffraction at 2.6Å (R-value: 23%). The subunits of PABP, RRM1 and RRM2, are examined in this article as the ''in vivo'' form seen in biological assembly 1 (via PDB). The protein has a homopolymeric structure, containing four RNA recognition motifs (RRMs), which are conserved. <ref name="Structure and Function">Kühn, Uwe and Elmar, Wahle. “Structure and Function of Poly(a) Binding Proteins.” Bba - Gene Structure & Expression, vol. 1678, no. 2/3, 2004. </ref> <scene name='78/782616/Rrm1_only/2'>RRM1</scene> and <scene name='78/782616/Rrm2_only/2'>RRM2</scene> are N-terminal domains that are connected by a <scene name='78/782616/Linker/3'>linker</scene>.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> Opposed to their counterparts, RRM3 and RRM4 bind Poly (A) RNA less tightly than RRM1 and RRM2.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> | The crystal structure PABP was derived from X-ray Diffraction at 2.6Å (R-value: 23%). The subunits of PABP, RRM1 and RRM2, are examined in this article as the ''in vivo'' form seen in biological assembly 1 (via PDB). The protein has a homopolymeric structure, containing four RNA recognition motifs (RRMs), which are conserved. <ref name="Structure and Function">Kühn, Uwe and Elmar, Wahle. “Structure and Function of Poly(a) Binding Proteins.” Bba - Gene Structure & Expression, vol. 1678, no. 2/3, 2004. </ref> <scene name='78/782616/Rrm1_only/2'>RRM1</scene> and <scene name='78/782616/Rrm2_only/2'>RRM2</scene> are N-terminal domains that are connected by a <scene name='78/782616/Linker/3'>linker</scene>.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> Opposed to their counterparts, RRM3 and RRM4 bind Poly (A) RNA less tightly than RRM1 and RRM2.<ref name="Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein">Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print. </ref> | ||
Revision as of 18:50, 6 April 2018
Contents |
Human Poly(A) Binding Protein (1CVJ)
Background
The Human Poly(A) Binding Protein (PABP) was discovered in 1973 by the use of a sedimentation profile detailing the RNase digestion differentiated the PABP protein. [1] Attempts to purify the 75 kDa protein then followed. In 1983, then considered “poly(A)-organizing protein,” was determined and purified by molecular weight, ligand-binding affinity, and amounts found in cytoplasmic portions of cell with ability to bind to free poly(A). [2]
PABP is a mRNA binding protein that binds to the 3’ Poly(A) tail on mRNA. It is comprised of four RNA recognition motifs (RRMs), which are highly conserved RNA-binding domains.[3] The RRM in PABP is found in over two hundred families of proteins across species, indicating that it is ancient.[3] Through extensive Adenosine recognition by the RRMs of PABP, the protein is involved in three main functions: recognition of the 3’ Poly(A) tail, mRNA stabilization, and eukaryotic translation initiation. The contributions of controlling gene expression via different families of PABPs is not yet fully understood. PABP families are divided into nuclear and cytoplasmic. [4] PABP1, which is predominantly cytoplasmic, is often referred to as PABP because it is the only form of PABP that has been extensively studied in its role with mRNA translation and stability. [4]
Structure
| |||||||||||
References
- ↑ Blobel, Gunter. “A Protein of Molecular Weight 78,000 Bound to the Polyadenylate Region of Eukaryotic Messenger Rnas.” Proceedings of the National Academy of Sciences of the United States of America, vol. 70, no. 3, 1973, pp. 924–8.
- ↑ Baer, Bradford W. and Kornberg, Roger D. "The Protein Responsible for the Repeating Structure of Cytoplasmic Poly(A)-Ribonucleoprotein." The Journal of Cell Biology, vol. 96, no. 3, Mar. 1983, pp. 717-721. EBSCOhost.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Deo, Rahul C, et al. “Recognition of Polyadenylate RNA by the Poly(A)-Binding Protein.” Cell 98:6. (1999) 835-845. Print.
- ↑ 4.0 4.1 4.2 4.3 4.4 Gorgoni, Barbra, and Gray, Nicola. “The Roles of Cytoplasmic Poly(A)-Binding Proteins in Regulating Gene Expression: A Developmental Perspective.” Briefings in Functional Genomics and Proteomics, vol. 3, no. 2, 1 Aug. 2004, pp. 125–141., doi:10.1093/bfgp/3.2.125.
- ↑ Kühn, Uwe and Elmar, Wahle. “Structure and Function of Poly(a) Binding Proteins.” Bba - Gene Structure & Expression, vol. 1678, no. 2/3, 2004.
- ↑ Wang, Zuoren and Kiledjian, Megerditch. “The Poly(A)-Binding Protein and an mRNA Stability Protein Jointly Regulate an Endoribonuclease Activity.” Molecular and Cellular Biology 20.17 (2000): 6334–6341. Print.
- ↑ 7.0 7.1 7.2 7.3 “Oculopharyngeal Muscular Dystrophy.” NORD (National Organization for Rare Disorders), rarediseases.org/rare-diseases/oculopharyngeal-muscular-dystrophy/.
- ↑ Richard, Pascale, et al. “Correlation between PABPN1 Genotype and Disease Severity in Oculopharyngeal Muscular Dystrophy.” Neurology, vol. 88, no. 4, 2016, pp. 359–365., doi:10.1212/wnl.0000000000003554.