Nuclear polyadenylated RNA-binding protein
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
=Relationship to other proteins= | =Relationship to other proteins= | ||
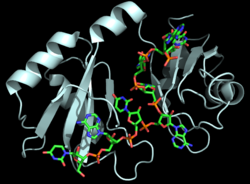
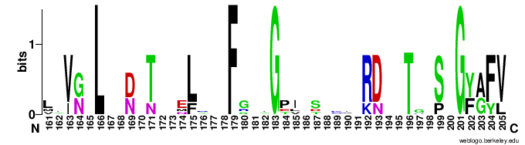
| - | The RNP-type RBD is found in many proteins involved in post-transcriptional [https://en.wikipedia.org/wiki/Post-transcriptional_modification pre-mRNA processing] (5'-end capping, splicing, 3'-end cleavage and polyadenylation, and transport from the nucleus)<ref name="RRMB">PMID: 18515081</ref>. The unique RBD of Hrp1 enables the protein to bind an RNA sequence that differs in both length and content from the RNA sequences of other RNA-binding and mRNA processing proteins such as [http://proteopedia.org/wiki/index.php/2sxl sex lethal], [https://en.wikipedia.org/wiki/Poly(A)-binding_protein Poly (A)-binding protein (PABP)], and [http://proteopedia.org/wiki/index.php/1fxl HuD] <ref name="GM3H"/>. These proteins, in addition to Hrp1 and | + | The RNP-type RBD is found in many proteins involved in post-transcriptional [https://en.wikipedia.org/wiki/Post-transcriptional_modification pre-mRNA processing] (5'-end capping, splicing, 3'-end cleavage and polyadenylation, and transport from the nucleus)<ref name="RRMB">PMID: 18515081</ref>. The unique RBD of Hrp1 enables the protein to bind an RNA sequence that differs in both length and content from the RNA sequences of other RNA-binding and mRNA processing proteins such as [http://proteopedia.org/wiki/index.php/2sxl sex lethal], [https://en.wikipedia.org/wiki/Poly(A)-binding_protein Poly (A)-binding protein (PABP)], and [http://proteopedia.org/wiki/index.php/1fxl HuD] <ref name="GM3H"/>. These proteins, in addition to Hrp1 and [https://blast.ncbi.nlm.nih.gov/Blast.cgi SRp20], contain conserved hydrophobic residues which contribute to hydrophobic interactions between secondary structures of the proteins. Each protein also contains conserved residues L166 and G201 which form a hydrogen bond, linking the β-sheets in the βαβ complex of Hrp1 (Figure 3). [[Image:Conserved_Hrp1_sequence_logo.png|525 px|center|thumb|Figure 3: Sequence logo for residues 161-205 of Hrp1.]] Like Hrp1, each of these proteins belongs to the class of single-stranded proteins composed of two canonical RBDs; however, each protein is differentiated by respective target RNA sequences, interactions with RNA at the atomic level, and interdomain contacts <ref name="GM3H"/>. Hrp1 is unique in that HuD, sex lethal, and PABP all contain at least one intra-RNA base-base stacking interaction, a feature that is lacking in the Hrp1-PEE complex <ref name="GM3H"/>. It is possible that the intra-RNA interactions found in these other protein-RNA complexes is replaced by the crucial Trp168-Ade4 stacking interaction found in the Hrp1 complex <ref name="GM3H"/>. This may help explain why the Hrp1-RNA interface involves only 6 nucleotides whereas PABP, sex lethal, and HuD require a longer 8-10 nucleotide sequence in the RNA binding pocket <ref name="GM3H"/>. |
</StructureSection> | </StructureSection> | ||
=References= | =References= | ||
<references/> | <references/> | ||
Revision as of 22:53, 24 April 2018
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 Perez-Canadillas JM. Grabbing the message: structural basis of mRNA 3'UTR recognition by Hrp1. EMBO J. 2006 Jul 12;25(13):3167-78. Epub 2006 Jun 22. PMID:16794580
- ↑ 2.0 2.1 2.2 2.3 2.4 Leeper TC, Qu X, Lu C, Moore C, Varani G. Novel protein-protein contacts facilitate mRNA 3'-processing signal recognition by Rna15 and Hrp1. J Mol Biol. 2010 Aug 20;401(3):334-49. Epub 2010 Jun 19. PMID:20600122 doi:10.1016/j.jmb.2010.06.032
- ↑ Kessler MM, Henry MF, Shen E, Zhao J, Gross S, Silver PA, Moore CL. Hrp1, a sequence-specific RNA-binding protein that shuttles between the nucleus and the cytoplasm, is required for mRNA 3'-end formation in yeast. Genes Dev. 1997 Oct 1;11(19):2545-56. PMID:9334319
- ↑ Clery A, Blatter M, Allain FH. RNA recognition motifs: boring? Not quite. Curr Opin Struct Biol. 2008 Jun;18(3):290-8. doi: 10.1016/j.sbi.2008.04.002. PMID:18515081 doi:http://dx.doi.org/10.1016/j.sbi.2008.04.002
Proteopedia Page Contributors and Editors (what is this?)
Cory A. Wuerch, Matthew Douglas Moore, Savannah Davis, Michal Harel, Jaime Prilusky