We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1451
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
== Function == | == Function == | ||
| - | Rhodopsin performs two functions. One function is to bind retinal. Rhodopsin is a protein that is essential for vision, especially in dim light. The photoreceptors in the retina that contain rhodopsin are rods. Rhodopsin is attached to 11-cis retinal which becomes excited by a photon of light and isomerizes to become all-trans conformation (NOTABILITY). This excitation activates rhodopsin and leads to depolarizing of neurons. The depolarizing of neurons is how the image is transmitted to the brain. (https://ghr.nlm.nih.gov/gene/RHO#) Another function is to function as a G protein-coupled receptor. When rhodopsin is activated by light the protein couples with the G protein transducin which is the first step in the signal cascade (PDF ON NOTABILITY) Rhodopsin must undergo several conformational changes before being able to bind transducin. (ALTERATIONS) Rhodopsin is initially converted to metarhodopsin II which is the active form of rhodopsin. Once the protein is active then metarhodopsin binds the G-protein tranducin and the GDP is exchanged for GTP. The G protein subunits dissociate and causes a decrease in cytosolic cGMP. The decrease in cGMP also causes the closing of calcium channels. When calcium concentrations drop the photoreceptors become hyperpolarized and this ensures the action potential is sent onward toward the brain. (RHODOPSIN AND RP). Photoreceptors are unable to be immediately restimulated, rhodopsin kinase phosphorylates the protein on serine and threonine residues which leads to the binding of arrestin. Arrestin prevents rhodopsin from interacting with tranducin again, thus preventing restimulation of the protein. Arrestin is released from the protein via phospotase A which dephosphorylates rhodopsin. (RHODOPSIN AND RP) | + | Rhodopsin performs two functions. One function is to bind retinal. Rhodopsin is a protein that is essential for vision, especially in dim light. The photoreceptors in the retina that contain rhodopsin are rods. Rhodopsin is attached to 11-cis retinal which becomes excited by a photon of light and isomerizes to become all-trans conformation (NOTABILITY). This excitation activates rhodopsin and leads to depolarizing of neurons. The depolarizing of neurons is how the image is transmitted to the brain. (https://ghr.nlm.nih.gov/gene/RHO#) Another function is to function as a G protein-coupled receptor. When rhodopsin is activated by light the protein couples with the G protein transducin which is the first step in the signal cascade (PDF ON NOTABILITY) Rhodopsin must undergo several conformational changes before being able to bind transducin. (ALTERATIONS) Rhodopsin is initially converted to metarhodopsin II which is the active form of rhodopsin. Once the protein is active then metarhodopsin binds the G-protein tranducin and the GDP is exchanged for GTP. The G protein subunits dissociate and causes a decrease in cytosolic cGMP. The decrease in cGMP also causes the closing of calcium channels. When calcium concentrations drop the photoreceptors become hyperpolarized and this ensures the action potential is sent onward toward the brain. (RHODOPSIN AND RP). Photoreceptors are unable to be immediately restimulated, rhodopsin kinase phosphorylates the protein on serine and threonine residues which leads to the binding of arrestin. Arrestin prevents rhodopsin from interacting with tranducin again, thus preventing restimulation of the protein. Arrestin is released from the protein via phospotase A which dephosphorylates rhodopsin. (RHODOPSIN AND RP) |
== Disease == | == Disease == | ||
| Line 34: | Line 34: | ||
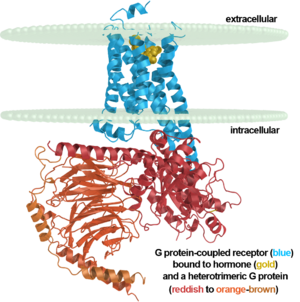
[[Image:7tm labeled.png|right|300px]] | [[Image:7tm labeled.png|right|300px]] | ||
Up until 2007, rhodopsin was the only GPCR that had a high-resolution crystal structure and was the basis for other GPCR structures. (https://www.ncbi.nlm.nih.gov/pubmed/24041646) Most G-protein coupled receptors are a target for pharmaceutical companies as the receptors are involved in a variety of physiological and pathophysiological processes. (ALTERATIONS) Most GPCRs bind ligands with an open domain. Rhodopsin and other vision proteins are unique as the proteins acquire ligands via transient pores in that open between the transmembrane helices of the GPCR. The use of transient pores allows thermal stability of the rhodopsin protein. (NOTABILITY) | Up until 2007, rhodopsin was the only GPCR that had a high-resolution crystal structure and was the basis for other GPCR structures. (https://www.ncbi.nlm.nih.gov/pubmed/24041646) Most G-protein coupled receptors are a target for pharmaceutical companies as the receptors are involved in a variety of physiological and pathophysiological processes. (ALTERATIONS) Most GPCRs bind ligands with an open domain. Rhodopsin and other vision proteins are unique as the proteins acquire ligands via transient pores in that open between the transmembrane helices of the GPCR. The use of transient pores allows thermal stability of the rhodopsin protein. (NOTABILITY) | ||
| - | [[Image:F4.large.jpg]] | ||
Revision as of 01:05, 29 April 2018
>
| This Sandbox is Reserved from Jan 22 through May 22, 2018 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, Missouri, USA. This reservation includes Sandbox Reserved 1446 through Sandbox Reserved 1455. |
To get started:
More help: Help:Editing |
| |||||||||||