This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1451
From Proteopedia
(Difference between revisions)
| Line 39: | Line 39: | ||
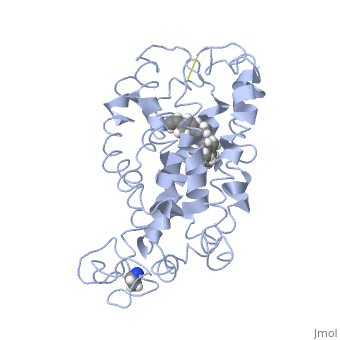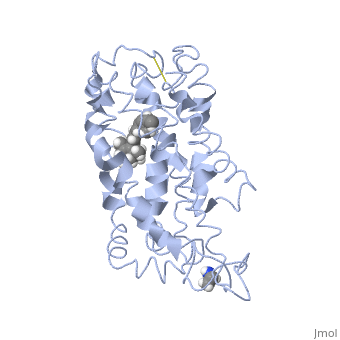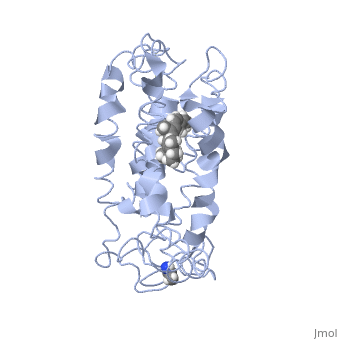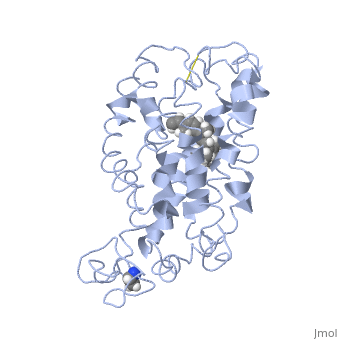
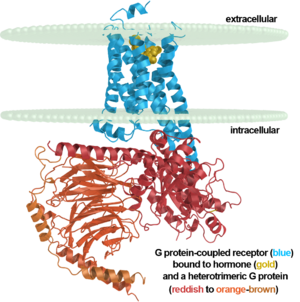
<scene name='77/778331/Rhodopsin/1'>Rhodopsin</scene> Fully functional rhodopsin has the typical GPCR structure of a seven transmembrane helical bundle with the N-terminus on the interior of the rods and the C-terminus in the cytoplasm. The N-terminus is located near the extracellular loops and ends of the transmembrane protein. There are hydrogen bonding between the transmembrane sections and the extracellular loops that are involved in the activation of rhodopsin when a photon is received. The N-terminus is thought to play a role in orientation of the extracellular loops<ref name="Article4">PMID:29042326</ref>. Transmembrane domain 1 and 2 play a role in stabilizing the protein and giving the protein functionality<ref name="Article3">PMID:21352497</ref>. Rhodopsin has two components: opsin (a membrane-bound polypeptide) and 11-cis-retinal (a chromophore that is bound to opsin via a protonated Schiff-base)<ref name="Article5">PMID:12402507</ref>. | <scene name='77/778331/Rhodopsin/1'>Rhodopsin</scene> Fully functional rhodopsin has the typical GPCR structure of a seven transmembrane helical bundle with the N-terminus on the interior of the rods and the C-terminus in the cytoplasm. The N-terminus is located near the extracellular loops and ends of the transmembrane protein. There are hydrogen bonding between the transmembrane sections and the extracellular loops that are involved in the activation of rhodopsin when a photon is received. The N-terminus is thought to play a role in orientation of the extracellular loops<ref name="Article4">PMID:29042326</ref>. Transmembrane domain 1 and 2 play a role in stabilizing the protein and giving the protein functionality<ref name="Article3">PMID:21352497</ref>. Rhodopsin has two components: opsin (a membrane-bound polypeptide) and 11-cis-retinal (a chromophore that is bound to opsin via a protonated Schiff-base)<ref name="Article5">PMID:12402507</ref>. | ||
| - | <scene name='77/778331/11-cis retinal/1'>11-cis retinal</scene> 11-cis retinal is the ligand, a molecule that is derived from vitamin A, is necessary for rhodopsin function. The ligand performs an inverse agonist suppressing activity on the photon receptor and is associated with the protein via protonated Schiff-bases linked to a lysine reside on the seventh domain | + | <scene name='77/778331/11-cis retinal/1'>11-cis retinal</scene> 11-cis retinal is the ligand, a molecule that is derived from vitamin A, is necessary for rhodopsin function. The ligand performs an inverse agonist suppressing activity on the photon receptor and is associated with the protein via protonated Schiff-bases linked to a lysine reside on the seventh domain<ref name="Article3">PMID:21352497</ref>. A negative agonist means the ligand, when present in the binding pocket of the protein, inhibits the receptor activity<ref name="Article5">PMID:12402507</ref>. The isomerization of cis to trans causes the protein complex to relax which allows for binding of transducin and the signal cascade to progress<ref name="Article3">PMID:21352497</ref>. |
<scene name='77/778331/Rhodopsin bound 11-cis retinal/1'>Rhodopsin bound 11-cis retinal</scene> This is rhodopsin with 11-cis retinal bound. After 11-cis retinal becomes activated and becomes all-trans, rhodopsin undergoes the conformational change to become metarhodopsin I and then metarhodopsin II which is the fully active form of rhodopsin. Metarhodopsin II then associates with the G protein transducin and the signal cascade can continue. | <scene name='77/778331/Rhodopsin bound 11-cis retinal/1'>Rhodopsin bound 11-cis retinal</scene> This is rhodopsin with 11-cis retinal bound. After 11-cis retinal becomes activated and becomes all-trans, rhodopsin undergoes the conformational change to become metarhodopsin I and then metarhodopsin II which is the fully active form of rhodopsin. Metarhodopsin II then associates with the G protein transducin and the signal cascade can continue. | ||
Revision as of 19:24, 30 April 2018
>
| This Sandbox is Reserved from Jan 22 through May 22, 2018 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, Missouri, USA. This reservation includes Sandbox Reserved 1446 through Sandbox Reserved 1455. |
To get started:
More help: Help:Editing |
| |||||||||||